Authors: Rijo M Choorakuttil, Palanisamy Devarajan, Bavaharan Rajalingam, Neelam Jain, Lalit K Sharma, Shweta Nagar, Akanksha Baghel, Neeraj Gupta, J.P. Chouhan, Ramesh S Shenoy, Praveen K Nirmalan for the Samrakshan Team.
Running Title: 1st Trimester screening and preterm PE
Author Affiliations
- Rijo M Choorakuttil, National Coordinator for Samrakshan IRIA, AMMA Center for Diagnosis and Preventive Medicine, Kochi, Kerala, India
- Palanisamy Devarajan, Nethra Scans and Genetic Clinic, Tiruppur, Tamil Nadu, India
- Rajalingam Bavaharan, Fetocare Magnum Imaging and Diagnostics, Trichy, Tamil Nadu, India
- Neelam Jain, Jain Ultrasound centre, C-112, B block , Dispensary road Sonari, Jamshedpur Jharkhand, India
- Lalit K Sharma, Raj Sonography & X- Ray Clinic, Baiju Choraha, Nayapura, Guna, Madhya Pradesh, India
- Shweta Nagar, Dr. Shweta Nagar’s Ultrasound Clinic & Imaging Centre, Flat no. 101, Princes Center, above Mama Loca cafe , 6/3 New Palasia, Indore, Madhya Pradesh, India
- Akanksha Baghel, Baghel Sonography Center. Front of Janpat Office, near District Hospital, Harda, Madhya Pradesh, India
- Neeraj Gupta, Neeraj Diagnostic And Imaging Center, near Surya hotel, South Tokoganj, Indore, Madhya Pradesh, India
- J. P. Chouhan, Purnima Hospital and Research Center, 74, Narmada Marg, Barwaha, Khargone, Madhya Pradesh, India
- Ramesh S Shenoy, Consultant Radiologist, Lisie Hospital, Ernakulam, Kerala, India
- Praveen K Nirmalan, Chief Research Mentor, AMMA ERF, AMMA Center for Diagnosis and Preventive Medicine, Kochi, Kerala, India
Corresponding Author: Rijo M Choorakuttil, National Coordinator for Samrakshan IRIA, AMMA Center for Diagnosis and Preventive Medicine, Kochi, Kerala, India. E-mail: rijomc@gmail.com
Key words: Pregnancy, 1st trimester, screening, pre eclampsia, India
Samrakshan, a nationwide program of the Indian Radiological & Imaging Association (IRIA) launched in June 2019, aims to optimize the experience and expertise of radiologists in India to supplement and complement existing efforts that address perinatal mortality in India. Samrakshan aims at a pragmatic approach cognizant of local realities and flexible that it can be adopted by many rather than a few.
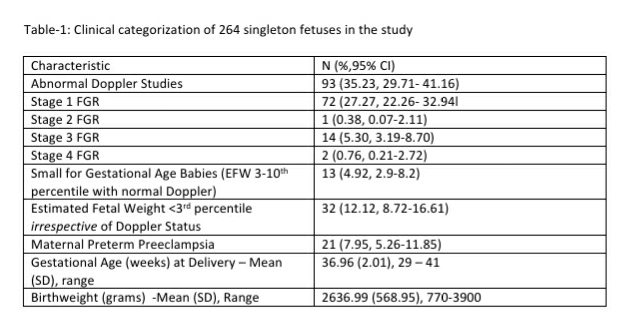
Pre-eclampsia (PE) and Fetal Growth Restriction (FGR) are the conditions of interest for Samrakshan. These conditions are important health problems in India as judged by its frequency and severity. The epidemiology, incidence, prevalence and natural history of these two conditions are reasonably understood from latent to declared disease and there is robust evidence about the association between several risk factors and the risk of serious or treatable disease.
The National Family Health Survey-4 (NFHS-4), a nationally representative survey carried out in 2015-16 in India, reported a perinatal mortality rate of 36 per 1000 pregnancies. [1] The perinatal mortality rate ranged from 8 per 1,000 pregnancies in Kerala to 56 per 1,000 pregnancies in Uttar Pradesh.[1] NFHS-4 reported a still birth rate of 0.7% and a neonatal mortality rate of 30 per 1000 live births in India, and a birth weight <2500 grams in 18.2% of live births.[1] A major proportion (41.77%) of neonatal deaths in India is attributable to prematurity and low birth weight.[2] The maternal mortality ratio in India has declined and is reported as 130 per 100,000 live births in 2014-16. [3] Pregnancy induced hypertension remains a major cause for maternal mortality, preterm deliveries, fetal growth restriction and low birth weight in India.
The NFHS-4 reported an increasing utilization of antenatal care (ANC) with 82.7% pregnant women reporting at least one antenatal care visit, however, only 58.6% women in the 1st trimester of pregnancy sought antenatal care.[1] Although an estimated 79% of all deliveries were institutional deliveries, only 61% of all pregnancies had received at least one ultrasound exam during pregnancy.[1]
In this manuscript, we discuss the protocol and the rationale of the 1st trimester opportunistic screening implemented through the Samrakshan program launched by IRIA, with a specific focus on PE .
Definitions
Standardizing measures and harmonizing definitions are integral to the long term success of screening programs. These allow comparisons with other programs and approaches and replication on a larger scale. The definition of PE and Pregnancy Induced Hypertension (PIH) is thus important. Samrakshan endorses the definition of PE and PIH [4] proposed by the International Society for the Study of Hypertension in Pregnancy (ISSHP).
Gestational hypertension is defined as persistent hypertension (systolic blood pressure ≥140 mm Hg and/or diastolic blood pressure ≥90 mm Hg after 20 weeks of gestation in the absence of features of PE. [4]
The ISSHP defines PE [4] as a systolic blood pressure at ≥140 mm Hg and/or diastolic blood pressure at ≥90 mm Hg on at least two occasions measured 4 hours apart in previously normotensive women and is accompanied by one or more of the following new‐onset conditions at or after 20 weeks of gestation:
- Proteinuria
- ≥30 mg/mol protein: creatinine ratio
- ≥300 mg/24 hour
- or ≥2 + dipstick
- Evidence of other maternal
organ dysfunction
- Acute kidney injury (creatinine ≥90 μmol/L; 1 mg/dL)
- Liver involvement (elevated transaminases, e.g. alanine aminotransferase or aspartate aminotransferase >40 IU/L) with or without right upper quadrant or epigastric abdominal pain
- Neurological complications (e.g. eclampsia, altered mental status, blindness, stroke, clonus, severe headaches, and persistent visual scotomata)
- or Haematological complications (thrombocytopenia–platelet count <150 000/μL, disseminated intravascular coagulation, haemolysis)
- Uteroplacental dysfunction
- Fetal growth restriction,
- Abnormal umbilical artery Doppler waveform analysis
- or Stillbirth
PE can be classified into four, not necessarily mutually exclusive, categories:
- Early‐onset PE (with delivery at <34+0 weeks of gestation)
- Preterm PE (with delivery at <37+0 weeks of gestation)
- Late‐onset PE (with delivery at ≥34+0 weeks of gestation)
- Term PE (with delivery at ≥37+0 weeks of gestation)
Superimposed PE on chronic hypertension is considered when women with chronic essential hypertension develop any of the above maternal organ dysfunctions consistent with pre‐eclampsia.[4] Increase in blood pressure in itself is not sufficient to diagnose superimposed PE. In the absence of pre‐existing proteinuria, new‐onset proteinuria in the setting of a rise in blood pressure is sufficient to diagnose superimposed PE.
Measurement of Blood Pressures
Instrumentation
Samrakshan recommends the use of validated digital automated or semi automated oscillometry machines to measure BP. Samrakshan recommends calibrating the machines at least once every 3-4 months. The use of digital oscillometry machines are recommended for several reasons that include
- Consistency with global protocols for the measurement of Blood pressure as part of trimester specific screening protocols for PE
- Environmental concerns with the use of mercury sphygmomanometer
- Availability and Affordability of Digital oscillometry machines to measure BP. These are now available on online shopping portals.
Method to measure BP [5]
The woman should be seated with their arms well supported at the level of their heart and an appropriate‐sized adult cuff (small <22 cm, normal 22–32 cm, or large 33–42 cm) used depending on the mid‐arm circumference. After rest for 5 minutes, blood pressure is measured in both arms simultaneously and two sets of recordings are made at 1‐minute intervals. As a pragmatic recommendation, BP maybe measured in one arm and two recordings are made at 1‐minute intervals. The four sets of systolic BP and diastolic BP measurements are then used to determine the Mean Arterial Pressure (MAP) using the following equation:
MAP= diastolic BP + (systolic BP-diastolic BP)/3.
The 4 sets of systolic and diastolic BP measures are input into the risk calculator and the final MAP measurement (average of four sets of measurements) is determined.
Measurement of Uterine Artery PI [6]
Route: A transabdominal ultrasound scan is preferred although a transvaginal approach can also be used.
Applicable GA range: 11+0 to 13+6 weeks of gestation (corresponding to fetal crown–rump length (CRL) of 42–84 mm). Gestational age must be determined from the measurement of the fetal CRL.
Measurements of Uterine Artery PI: Samrakshan recommends the use of a standardized protocol, as advised by the Fetal Medicine Foundation and the International Society of Ultrasound in Obstetrics and Gynaecology [6] and quoted below.
“Obtain a sagittal section of the uterus is obtained and identify the cervical canal and internal cervical os. Keep the transducer in the midline and gently tilted to the side and use colour flow mapping to identify each uterine artery along the side of the cervix and uterus at the level of the internal os. Use Pulsed‐wave Doppler with the sampling gate set at 2 mm to cover the whole vessel. Take care to ensure that the angle of insonation is less than 30°. The uterine artery PI (UTPI) is measured when three similar consecutive waveforms are obtained and the mean UTPI of the left and right arteries is calculated”
The measurement of UTPI at the level of the internal os during the first trimester is more reproducible and can be achieved in a greater proportion of women than those obtained at the level of the crossover of the external iliac vessels. [7]
Additional Information from same test: The same scan is utilized for the measurement of fetal translucency thickness and diagnosis of any major fetal defects
The Screening Protocol
Applicable Gestational Age
- Gestational Age 11-13+6 weeks
Unique ID
- Assign each woman an unique ID that can be used to track progress and to document findings through the course of the pregnancy.
Assign an expected date of delivery (EDD)
- Ask the LMP of the woman. Please remember to explain to the woman that LMP is calculated from the 1st day of the last menstrual period
- Do a dating scan (if not done earlier)
- Measure Fetal Crown Rump Length
- Check for discrepancy in the EDD based on LMP and USG
- Assign an EDD
- Do not change the assigned EDD later during the course of the pregnancy. If the assigned EDD is changed, document clearly the reasons for the change
Clinico-Demographic Details
- Maternal Age in years
- Maternal Height in centimetres
- Maternal Weight in kilograms
- Ethnicity of the mother: Asian Indian/ Mixed
Past Obstetric History
- Parity nulliparous, parous without prior PE, parous with prior PE
- Interpregnancy interval in years between the birth of the last child
- Gestational age at previous childbirth/delivery (weeks)
- Birthweight of previous pregnancy beyond 24 weeks
Family History
- Family history of PE (mother)
Current Conception
- Method of conception: spontaneous, ovulation induction, in vitro fertilization
Personal Risk Behaviour:
- Smoking habit (mark yes if tobacco consumption is there in any form- ask for oral chewable forms of tobacco)
Co morbidities:
- History of chronic hypertension
- History of diabetes mellitus: type 1, type 2, insulin intake
- History of systemic lupus erythematosus or antiphospholipid syndrome
Blood Pressure Measures
- Systolic BP in both arms
- Diastolic BP in both arms
- Enter BP in the online risk calculator available online at https://fetalmedicine.org/research/assess/preeclampsia/first-trimester
- Estimate MAP using online calculator
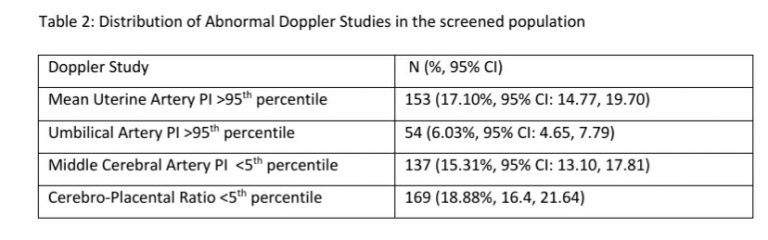
Doppler Measures
- Measure Right Uterine Artery PI
- Measure Left Uterine Artery PI
- Estimate Mean Uterine Artery PI
Biochemical Markers: Placental Growth Factor (PLGF) [8] and/or Pregnancy Associated Plasma Protein A (PAPP-A) [9] are biochemical markers that improve the detection rate of pregnant women at high risk for the development of preterm PE.
Samrakshan acknowledges that these tests are not widely available, accessible or affordable for the larger population of pregnant women in India. As a pragmatic measure, Samrakshan recommends that these tests may be done where possible but does not mandate the use of these tests.
Elements of the screening protocol
Each of the risk factors in the screening protocol are based on prior evidence for the association of these risk factors with PE [10-14] and hence we are not going into a detailed description, in this manuscript, of the association of the components of the screening protocol with onset of PE.
The rationale of choosing this screening protocol
In routine clinical practice, a common approach to determine the risk of PE is based on maternal demographics and medical history pertaining to maternal risk factors. [15-18] The National Institute for Health and Clinical Excellence (NICE) in the UK, issued recommendations that women should be considered to be at high risk of developing PE if they have any one high‐risk factor (hypertensive disease in previous pregnancy, chronic hypertension, chronic renal disease, diabetes mellitus, or autoimmune disease) or any two moderate‐risk factors (nulliparity, age ≥40 years, BMI ≥35 kg/m2, family history of PE, or interpregnancy interval >10 years).[12] In the USA, the American College of Obstetricians and Gynecologists (ACOG) recommended daily low‐dose aspirin starting from the late first trimester for women with a history of early‐onset PE and preterm delivery at less than 34 weeks of gestation, or for women with more than one prior pregnancy complicated by PE. [19] However, the detection rate is 39% for preterm PE and 34% for term PE at 10.3% false‐positive rate on screening using the NICE guidelines [20] and 90% and 89%, at 64.3% false‐positive rate using the US guidelines.[21]
The identification of four potentially useful biomarkers a) MAP, b) Uterine Artery Doppler studies c) PAPP A and d) PLGF has improved the effectiveness of screening protocols for PE. The detection rates of preterm PE and term PE improved to 75% and 43%, respectively, at false‐positive rate of 10% with the use of these biomarkers. [22,23]
The screen‐positive rate by the NICE method was 10.3%, the detection rate for all PE was 30%, and for preterm PE it was 41% in a recent study by the National Institute for Health Research (UK) that prospectively looked at validating the Bayesian based competing risk models that form the basis of the screening protocol Samrakshan is adopting. [24] The detection rate of the mini‐combined test (maternal factors, MAP, and PAPP‐A) for all PE was 43% and was superior to that of the NICE method by 12.1% (95% CI, 7.9–16.2). [24] In screening for preterm PE by a combination of maternal factors, MAP, UTPI, and PLGF, the detection rate was 82%, which was higher than that of the NICE method by 41.6% (95% CI, 33.2–49.9). [24]
What does Samrakshan recommend as the screening protocol?
- Samrakshan recommends a risk‐based screening for preterm PE using various biomarkers based on the available global evidence.
- Current evidence indicates that a checklist approach based on maternal history and risk factors alone is no longer sufficient to identify pregnant women at high risk for preterm PE.
- The competing risk Bayesian based algorithm or model has a higher detection rate.
- The detection rates of preterm PE will be reduced in the absence of biochemical biomarkers, PLGF, however, currently it is a pragmatic reality in India that biochemical biomarkers have to be optional given issues with affordability, accessibility and availability.
- Samrakshan recommends a paradigm shift to a one step 1st trimester screening including MAP and Uterine Artery PI Doppler studies for all pregnant women at 11-13+6 weeks
- Samrakshan considers a woman as high risk when the risk is 1 in 150 or more based on the first‐trimester combined test with maternal risk factors, MAP, and UTPI.
- Samrakshan recommends screening in the 1st trimester as an evidence based prophylactic intervention (low dose aspirin at 150mg once daily at bedtime) can be provided to pregnant women identified as at high risk for preterm PE until 36 weeks of gestation, or when delivery occurs, or when PE is diagnosed.[25] The prophylactic benefits of low dose aspirin will be discussed in a separate manuscript.
References
- International Institute for Population Sciences (IIPS) and ICF. 2017. National Family Health Survey (NFHS-4) 2015-16: India. Mumbai: IIPS
- Million Death Study Collaborators, Bassani DG, Kumar R, et al. Causes of neonatal and child mortality in India: a nationally representative mortality survey. Lancet. 2010; 376:1853–1860.
- Niti Ayog, India. Maternal Mortality Ratio (per 100,000 live births). Accessed online from https://www.niti.gov.in/content/maternal-mortality-ratio-mmr-100000-live-births, on September 2, 2019
- Brown MA, Magee LA, Kenny LC, et al. The hypertensive disorders of pregnancy: ISSHP classification, diagnosis & management recommendations for international practice. Pregnancy Hypertens. 2018; 13: 291– 310.
- Poon LC, Zymeri NA, Zamprakou A, Syngelaki A, Nicolaides KH. Protocol for measurement of mean arterial pressure at 11‐13 weeks’ gestation. Fetal Diagn Ther. 2012; 31: 42– 48.
- Sotiriadis A, Hernandez‐Andrade E, da Silva Costa F, et al. ISUOG Practice Guidelines: Role of ultrasound in screening for and follow‐up of pre‐eclampsia. Ultrasound Obstet Gynecol. 2019; 53: 7– 22.
- Lefebvre J, Demers S, Bujold E, et al. Comparison of two different sites of measurement for transabdominal uterine artery Doppler velocimetry at 11‐13 weeks. Ultrasound Obstet Gynecol. 2012;40:288–292.
- Zhong Y, Zhu F, Ding Y. Serum screening in first trimester to predict pre‐eclampsia, small for gestational age and preterm delivery: Systematic review and meta‐analysis. BMC Pregnancy Childbirth. 2015;15:191.
- Morris RK, Bilagi A, Devani P, Kilby MD. Association of serum PAPP‐A levels in first trimester with small for gestational age and adverse pregnancy outcomes: Systematic review and meta‐analysis. Prenat Diagn. 2017; 37: 253– 265
- Magee LA, Pels A, Helewa M, Rey E, von Dadelszen P; Canadian Hypertensive Disorders of Pregnancy (HDP) Working Group. Diagnosis, evaluation, and management of the hypertensive disorders of pregnancy. Pregnancy Hypertens. 2014; 4: 105– 145.
- Lowe SA, Bowyer L, Lust K, et al. SOMANZ guidelines for the management of hypertensive disorders of pregnancy 2014. Aust N Z J Obstet Gynaecol. 2015; 55: e1– e29.
- National Collaborating Centre for women’s and Children’s Health (UK). Hypertension in Pregnancy: The Management of Hypertensive Disorders During Pregnancy. London: RCOG Press; 2010.
- Lowe SA, Brown MA, Dekker GA, et al. Guidelines for the management of hypertensive disorders of pregnancy 2008. Aust N Z J Obstet Gynaecol. 2009;49:242–246.
- Khalil A, Syngelaki A, Maiz N, Zinevich Y, Nicolaides KH. Maternal age and adverse pregnancy outcome: A cohort study. Ultrasound Obstet Gynecol. 2013;42:634–643.
- Tranquilli AL, Dekker G, Magee L, et al. The classification, diagnosis and management of the hypertensive disorders of pregnancy: A revised statement from the ISSHP. Pregnancy Hypertens. 2014;4:97–104.
- World Health Organization. WHO Recommendations for Prevention and Treatment of pre‐Eclampsia and Eclampsia. Geneva: WHO; 2011.
- Bibbins‐Domingo K, Grossman DC, Curry SJ, et al. Screening for preeclampsia: US Preventive Services Task Force Recommendation Statement. JAMA. 2017;317:1661–1667.
- Committee Opinion No. 638: First‐trimester risk assessment for early‐onset preeclampsia. Obstet Gynecol. 2015;126:e25–e27.
- ACOG Committee Opinion No. 743 Summary: Low‐dose aspirin use during pregnancy. Obstet Gynecol. 2018;132:254–256.
- Wallenburg HC. Prevention of pre‐eclampsia: Status and perspectives 2000. Eur J Obstet Gynecol Reprod Biol. 2001;94:13–22.
- O’Gorman N, Wright D, Poon LC, et al. Multicenter screening for pre‐eclampsia by maternal factors and biomarkers at 11–13 weeks’ gestation: Comparison with NICE guidelines and ACOG recommendations. Ultrasound Obstet Gynecol. 2017;49:756–760.
- O’Gorman N, Wright D, Poon LC, et al. Accuracy of competing‐risks model in screening for pre‐eclampsia by maternal factors and biomarkers at 11‐13 weeks’ gestation. Ultrasound Obstet Gynecol. 2017;49:751–755.
- Rolnik DL, Wright D, Poon LCY, et al. ASPRE trial: Performance of screening for preterm pre‐eclampsia. Ultrasound Obstet Gynecol. 2017;50:492–495.
- Tan MY, Wright D, Syngelaki A, et al. Comparison of diagnostic accuracy of early screening for pre‐eclampsia by NICE guidelines and a method combining maternal factors and biomarkers: Results of SPREE. Ultrasound Obstet Gynecol. 2018;51:743–750.
- Rolnik DL, Wright D, Poon LC, et al. Aspirin versus placebo in pregnancies at high risk for preterm preeclampsia. N Engl J Med. 2017;377:613–622.