Author: Dr Shanas KP, KREST Fellow in Fetal Radiology and Fetal Medicine, AMMA Scans, AMMA Center for Diagnosis and Preventive Medicine Pvt Ltd, Kochi, Kerala
INTRODUCTION:
Fetal growth restriction (FGR) which occurs in 10 % of pregnancies is defined as the failure of the fetus to reach its genetically determined growth potential. (1). The identification of growth-restricted fetuses during pregnancy is important to ensure optimal monitoring and timing of birth and reduce the high risk of stillbirth and perinatal morbidity associated with FGR (2, 3). Screening for FGR and Pre-eclampsia (PE) and early administration of low dose Aspirin at 11-14 gestation weeks to women identified at high risk has significantly reduced the development of PE (4) and FGR. Radiologists must focus on accurate dating and integrate first-trimester screening in their practice to identify pregnancies at high risk for PE and FGR and to initiate preventative therapy at the appropriate time. Additionally, all pregnant women must be educated on the need for first-trimester scans as the uptake of first-trimester ultrasound scans in India is still suboptimal.
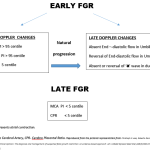
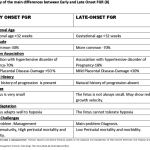
Currently, the criteria to define FGR are those derived from an international Delphi consensus (5). These criteria include biometric parameters and Doppler indices of fetoplacental function, which allow the classification of FGR into two phenotypes, early- and late-onset, based on the timing of diagnosis with a 32-gestational weeks cut-off. The gestational age at diagnosis is used to subclassify suspected fetal growth restriction into early and late, depending on whether the condition is diagnosed before or after 32 weeks of gestation. Early FGR is associated with umbilical artery Doppler abnormalities, whereas late FGR is often associated with a low pulsatility index in the middle cerebral artery. (6). Early and late FGR differ significantly in many aspects, such as prevalence, perinatal outcomes, and concomitant maternal diseases. Early FGR is frequently associated with hypertensive disorders of pregnancy (HDP), including gestational hypertension and preeclampsia compared to late-onset FGR.
This position paper aims to highlight the importance of accurate gestational age estimation and the appropriateness of the gestational age cut-off used for the classification of FGR based on available evidence from the scientific literature.
Evidence that supports the use of 32 gestational weeks as the cut-off to classify Early and Late FGR:
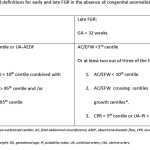
A Delphi survey was conducted among an international panel of 106 experts on FGR. The panel members were provided with 18 parameters based on the scientific literature to define FGR and were asked to rate the importance of these parameters for the diagnosis of both early and late FGR on a 5-point Likert scale. The possible algorithms to define early and late FGR were presented to the panel as two multiple-choice questions. The algorithm that received the most support was considered as the final one for consensus-based definitions. (5). The expert panel chose 32 gestational weeks as the cutoff to differentiate early onset and late-onset FGR.
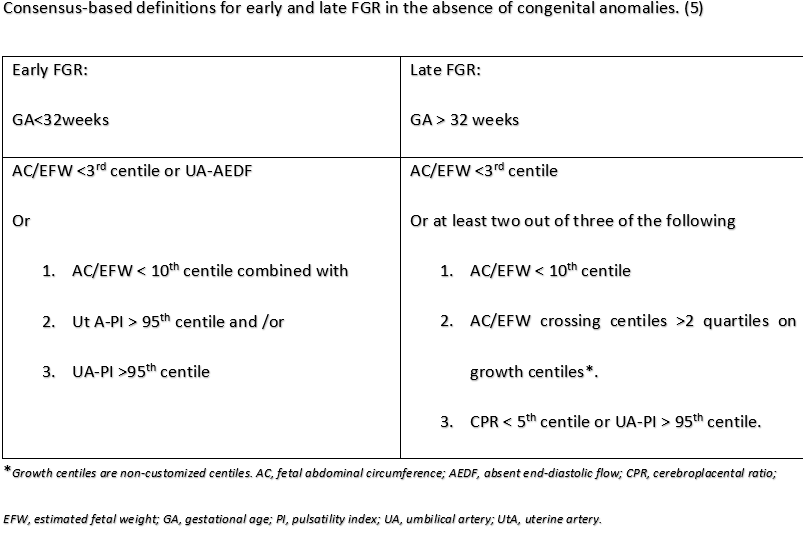
Another study in a cohort of 656 consecutive singleton pregnancies with FGR used a decision tree analysis to evaluate the GA cut-off that best discriminated perinatal mortality, association with PE and adverse perinatal outcome (fetal demise, early neonatal death, neonatal acidosis at birth, and 5-min Apgar score <7). The study investigators concluded that Umbilical artery (UA) Doppler discriminated better the two forms of FGR with early- and late-onset presentation, higher association with PE and poorer outcome. In the absence of UA information, a GA cut-off of 32 weeks at diagnosis maximizes differences between early- and late-onset FGR. (7)
By definition, any cut-off used to classify according to the gestational age at onset of FGR will be arbitrary and determined by the use and timing of third-trimester ultrasound in each setting, and by the protocol determining management and timing of delivery. In addition, since this is not an etiologic classification, it will be hampered by a huge degree of overlapping in clinical features (8).
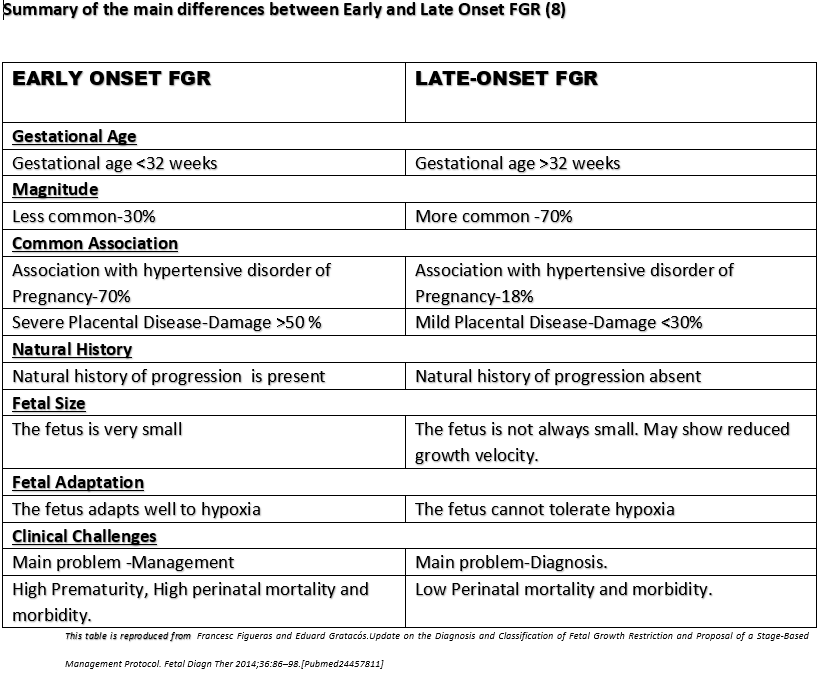
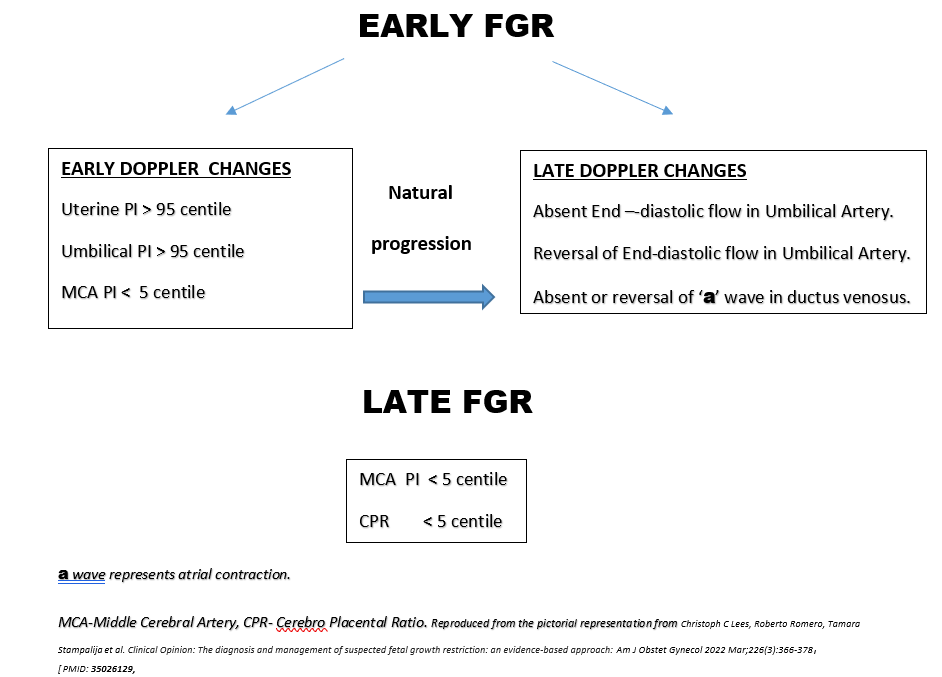
Evidence against the use of 32 weeks as the cut-off to classify Early and Late FGR:
An observational, prospective multicenter study conducted at the Obstetric Unit of three Italian university maternal-fetal referral centres in Lombardy and Tuscany (Milan, Monza and Florence) over a continuous period from November 2019 to April 2021 showed that Hypertensive Disorder of Pregnancy (HDP), rather than GA at diagnosis of FGR, identifies specific maternal hemodynamic patterns that distinguish two different FGR phenotypes(9). Of note, the ISUOG guidelines have for the first time included the maternal hemodynamic status in this classification, suggesting a more marked hemodynamic impairment characterized by low CO (Cardiac output) and high SVR (Systemic Vascular resistance) in early- versus late-onset FGR (3). The study investigators concluded that the presence of HDP, rather than the mere temporal variable of GA at FGR diagnosis, is a more accurate criterion to appreciate specific maternal hemodynamic patterns associated with FGR and to adequately distinguish different FGR phenotypes. (9) In addition, the classificatory analysis based on the Random Forest algorithm has shown that the classification of these high-risk pregnancies should include not just a single parameter, such as the GA at FGR diagnosis, but several variables, among which those related to maternal phenotype, i.e., BMI, and hemodynamic status are the most relevant. (9)
HDP-Hypertensive Disorder of Pregnancy, FGR-Fetal Growth Restriction, CO-Cardiac Output, SVR-Systemic Vascular Resistance
A retrospective cohort study on 895 women with singleton gestation who gave birth to a neonate diagnosed as small-for-gestational-age (small-for-gestational age, defined as birthweight <10th percentile for gestational age) at a tertiary referral centre between January 2001 and December 2015, and for whom placental pathology was available. The investigators aimed to use placental pathology findings to determine the optimal gestational age cut-off between early and late fetal growth restriction. The only histological finding that changed with gestational age was MVM (Maternal Vascular Malperfusion) pathology, which decreased in frequency with increasing gestational age. The investigators identified a considerable drop in the rate of MVM lesions at 33 weeks of gestation. The rate of MVM pathology in the placentas of infants born before 330/7 weeks was significantly higher than that observed in the placentas of infants born at 330/7 weeks or longer. Using placental pathology as a direct measure of the mechanisms underlying fetal growth restriction, the optimal gestational age at birth cut-off which differentiates early from late fetal growth restriction appears to be 330/7 weeks. (10).
MVM -Maternal Vascular Malperfusion .
A case-control study was performed at the high-risk clinic of the Maternal-Fetal Medicine Unit, tertiary referral centre, University of Milan from 1st May 2019 to 31st December 2021. The study included singleton pregnancies with HDP and normally grown fetuses (HDP-AGA fetuses), with HDP and FGR, early FGR, late FGR, and uneventful pregnancies. The investigators found a high prevalence of placental MVM in HDP-FGR and early FGR groups. These lesions were also associated with abnormal, anti-, and angiogenic markers. Conversely, HDP-AGA fetuses and late FGR presented more heterogeneous placental lesions not severe enough to cause fetoplacental Doppler anomalies. (11). This study highlights the significance of placental pathology in the differentiation of both phenotypes.
HDP-Hypertensive Disorder of Pregnancy, AGA-Appropriate for Gestational Age.
CONCLUSION:
The pathogenesis of the two forms of FGR differs and it is the histopathological findings of the placenta that best differentiates the two forms. Ultrasound (US) is the gold standard for non-ionizing non-invasive imaging used to assess placental structure. However, the US alone is not sufficient to fully characterize placental pathophysiology, including vascular and metabolic functions and their relationship to clinical maternal and fetal outcomes (12). So, the determination of an exact gestational age for the differentiation of FGR is not truly possible. However, it is more important to diagnose FGR to prevent short-term and long-term neurodevelopmental sequelae. Diagnosis of FGR is possible only by accurate dating of pregnancy in the first trimester and the major limitations in India are an inaccurate recall of LMP, lack of uptake of first trimester scan and failure to practice the exact guidelines for dating. As placental histopathology is a retrospective evaluation, it cannot help with the management of the present pregnancy. Thus considering these limitations, Doppler surveillance with accurate dating of gestational age may be a more viable option at this stage to accurately identify fetuses at increased risk of perinatal mortality and morbidity. A gestational age cut-off can help to prognosticate the two forms and may aid clinicians in decision-making regarding proper surveillance protocols for FGR including frequency of follow-up visits, ultrasound and Doppler studies, and timing of corticosteroids and childbirth. Early FGR is associated with a high risk of prematurity and fetuses with genetic abnormalities can also present with early-onset FGR, commonly in association with fetal and amniotic fluid abnormalities (13) whereas late FGR is associated with better prognosis. Hence, the cut-off to define early- versus late-onset FGR has commonly been set arbitrarily at about 32–34 weeks at diagnosis or 37 weeks at delivery(8). The choice of 32 weeks as the cutoff value between early and late FGR appears appropriate because hypertrophy of fetal cells initiates approximately at this GA (14). So, rather than finding a gestational age cut-off, it is important to carry out proper surveillance with Doppler parameters(Umbilical and Uterine arteries are indirect indicators of the fetal and maternal side of the placenta and MCA, CPR being parameters of fetal adaptive response to hypoxia) for prompt identification of fetuses at increased risk and to determine the accurate timing of delivery for them to reduce preterm births and perinatal mortality and morbidity.
REFERENCES
- Unterscheider J, Daly S, Geary MP, Kennelly MM, McAuliffe FM, O’Donoghue K, et al. Optimizing the definition of intrauterine growth restriction: the multicenter prospective PORTO Study. Am J Obstet Gynecol. 2013 Apr;208(4):290.e1-6. doi: 10.1016/j.ajog.2013.02.007. PMID: 23531326. [Pubmed:23531326].
- Lees CC, Marlow N, van Wassenaer-Leemhuis A, Arabin B, Bilardo CM, Brezinka C, et al; TRUFFLE study group. 2 year neurodevelopmental and intermediate perinatal outcomes in infants with very preterm fetal growth restriction (TRUFFLE): a randomised trial. Lancet. 2015 May 30;385(9983):2162-72. doi: 10.1016/S0140-6736(14)62049-3. Epub 2015 Mar 5. Erratum in: Lancet. 2015 May 30;385(9983):2152. PMID: 25747582.
- Lees CC, Stampalija T, Baschat A, da Silva Costa F, Ferrazzi E, Figueras F, et al. ISUOG Practice Guidelines: diagnosis and management of small-for-gestational-age fetus and fetal growth restriction. Ultrasound Obstet Gynecol. 2020 Aug;56(2):298-312. doi: 10.1002/uog.22134. PMID: 32738107.
- Rolnik DL, Wright D, Poon LCY, Syngelaki A, O’Gorman N, de Paco Matallana C, et al. ASPRE trial: performance of screening for preterm pre-eclampsia. Ultrasound Obstet Gynecol. 2017 Oct;50(4):492-495. doi: 10.1002/uog.18816. Epub 2017 Aug 24. Erratum in: Ultrasound Obstet Gynecol. 2017 Dec;50(6):807. PMID: 28741785.
- Gordijn SJ, Beune IM, Thilaganathan B, Papageorghiou A, Baschat AA, Baker PN, et al. Consensus definition of fetal growth restriction: a Delphi procedure. Ultrasound Obstet Gynecol. 2016 Sep;48(3):333-9. doi: 10.1002/uog.15884. PMID: 26909664.
- Lees CC, Romero R, Stampalija T, Dall’Asta A, DeVore GA, Prefumo F, et al Clinical Opinion: The diagnosis and management of suspected fetal growth restriction: an evidence-based approach. Am J Obstet Gynecol. 2022 Mar;226(3):366-378. doi: 10.1016/j.ajog.2021.11.1357. Epub 2022 Jan 10. PMID: 35026129; PMCID: PMC9125563.
- Savchev S, Figueras F, Sanz-Cortes M, Cruz-Lemini M, Triunfo S, Botet F, et al. Evaluation of an optimal gestational age cut-off for the definition of early- and late-onset fetal growth restriction. Fetal Diagn Ther. 2014;36(2):99-105. doi: 10.1159/000355525. Epub 2013 Nov 6. PMID: 24217372.
- Figueras F, Gratacós E. Update on the diagnosis and classification of fetal growth restriction and proposal of a stage-based management protocol. Fetal Diagn Ther. 2014;36(2):86-98. doi: 10.1159/000357592. Epub 2014 Jan 23. PMID: 24457811.
- Ornaghi S, Caricati A, Di Martino DD, Mossa M, Di Nicola S, Invernizzi F, et al. Non-invasive Maternal Hemodynamic Assessment to Classify High-Risk Pregnancies Complicated by Fetal Growth Restriction. Front Clin Diabetes Healthc. 2022 May 4;3:851971. doi: 10.3389/fcdhc.2022.851971. PMID: 36992751; PMCID: PMC10012115.
- Aviram A, Sherman C, Kingdom J, Zaltz A, Barrett J, Melamed N. Defining early vs late fetal growth restriction by placental pathology. Acta Obstet Gynecol Scand. 2019 Mar;98(3):365-373. doi: 10.1111/aogs.13499. Epub 2018 Nov 22. PMID: 30372519.
- Di Martino DD, Avagliano L, Ferrazzi E, Fusè F, Sterpi V, Parasiliti M, et al. Hypertensive Disorders of Pregnancy and Fetal Growth Restriction: Clinical Characteristics and Placental Lesions and Possible Preventive Nutritional Targets. Nutrients. 2022 Aug 10;14(16):3276. doi: 10.3390/nu14163276. PMID: 36014782; PMCID: PMC9414322.
- Nguyen CD, Correia-Branco A, Adhikari N, Mercan E, Mallidi S, Wallingford MC. New Frontiers in Placenta Tissue Imaging. EMJ Radiol. 2020 Sep;1(1):54-62. doi: 10.33590/emjradiol/19-00210. PMID: 35949207; PMCID: PMC9361653.
- Robert Resnik .Intrauterine growth restriction . Obstet Gynecol .2002 Mar;99(3):490-6 [PMID: 11864679].
- Nardozza LMM, Zamarian ACP, Araujo Júnior E. New Definition of Fetal Growth Restriction: Consensus Regarding a Major Obstetric Complication. Rev Bras Ginecol Obstet. 2017 Jul;39(7):315-316. doi: 10.1055/s-0037-1603741. Epub 2017 Jun 12. PMID: 28605820; PMCID: PMC10416171.
- J Kingdom, B Huppertz, G Seaward, P Kaufmann. Development of the placental villous tree and its consequences for fetal growth. Eur J Obstet Gynecol Reprod Biol. 2000 Sep;92(1):35-43 [PMID: 10986432].
- E. Roma, A. Arnau, R. Berdala, C. Bergos, J. Montesinos, F. Figueras. Ultrasound screening for fetal growth restriction at 36 vs32 weeks’ gestation: a randomized trial (ROUTE). Ultrasound Obstet Gynecol.2015 Oct;46(4):391-7. [PMID: 26031399].
- Burton GJ, Jauniaux E. Pathophysiology of placental-derived fetal growth restriction. Am J Obstet Gynecol. 2018 Feb;218(2S):S745-S761. doi: 10.1016/j.ajog.2017.11.577. PMID: 29422210.
- Bamberg C, Kalache KD. Prenatal diagnosis of fetal growth restriction. Semin Fetal Neonatal Med. 2004 Oct;9(5):387-94. doi: 10.1016/j.siny.2004.03.007. PMID: 15691774.
- Audette MC, Kingdom JC. Screening for fetal growth restriction and placental insufficiency. Semin Fetal Neonatal Med. 2018 Apr;23(2):119-125. doi: 10.1016/j.siny.2017.11.004. Epub 2017 Dec 6. PMID: 29221766.
- Kingdom JC, Audette MC, Hobson SR, Windrim RC, Morgen E. A placenta clinic approach to the diagnosis and management of fetal growth restriction. Am J Obstet Gynecol. 2018 Feb;218(2S):S803-S817. doi: 10.1016/j.ajog.2017.11.575. Epub 2017 Dec 15. PMID: 29254754.