Author: Dr Nivedita Biradar, KREST Fellow in Fetal Radiology and Fetal Medicine, Delta Scans, Bengaluru
Pre-eclampsia and other hypertensive disorders in pregnancy are a major cause of maternal and perinatal mortality and morbidity globally. Globally, the incidence of HDP has increased from 16.30 million to 18.08 million from 1990 to 2019, a total increase of 10.9% over two decades. (1). Pre-eclampsia is responsible for >70,000 maternal deaths each year around the world (2) which translates to one woman dying every 7 minutes. Due to its syndromic nature and varying clinical presentations of preeclampsia phenotypes (3), the reliability and specificity with which we can predict pre-eclampsia in women is a challenge. However, the Prediction of preeclampsia has the potential to prevent the overdiagnosis and/or undertreatment of pregnant women and even allow effective perinatal outcomes and even efficient utilization of healthcare resources. The prevalence of PE can potentially be halved by a strategy of early identification of the high-risk group and the prophylactic use of low-dose aspirin. (4,5)
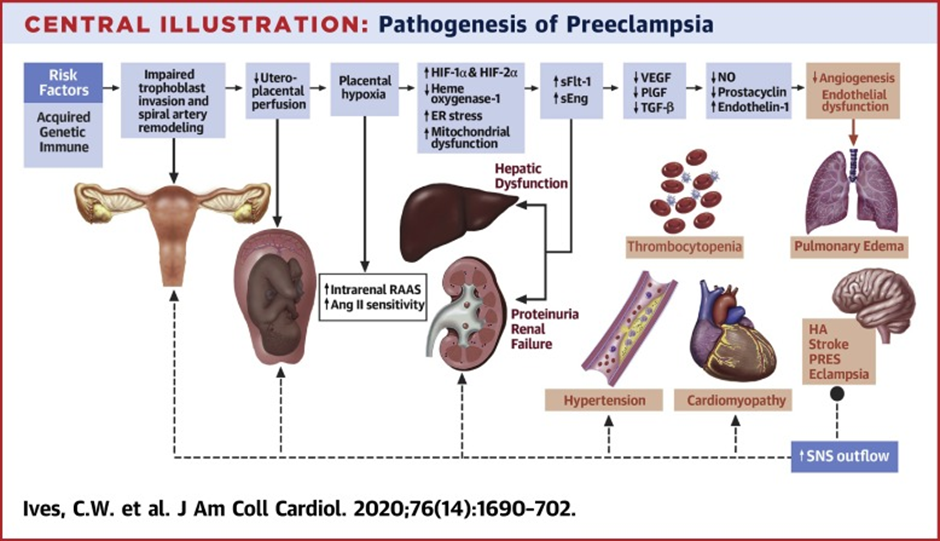
During the process of placentation, which usually happens in 3 waves the first one being around 8-10weeks, the second wave at 16-18weeks and the third wave by about 24 weeks, the trophoblasts invade and transform the spiral arterioles causing high resistance vessels to change to high capacitance and low resistance vessels but in Preeclampsia there is an incomplete trophoblastic invasion of spiral arterioles, as a result, these arteries continue to show high resistance to the flow of blood. (6)
The criteria for diagnosing preeclampsia includes two blood pressure readings at least 4-6 h apart that are greater than 140/90 occurring after 20 weeks gestation in a woman not known to be previously hypertensive (7) and proteinuria ≥300 mg in 24 hours or one reading of at least ‘+’ on dipstick analysis of midstream urine specimen. The recent proposition was that early and late PE are not two different diseases, but rather that PE is a spectrum disorder. (8)
The prevalence of PE can be decreased if not eliminated, through early identification of pregnant women who are at high risk of development of PE and prophylactic use of aspirin. (5,9) Various screening models have been developed by WHO, NIH, and ACOG that can help in screening pregnant women by using maternal characteristics and medical history with the results of various combinations of biophysical(MAP and mean uterine artery PI) and biochemical measurements(PLGF, PAPPA). (8,10,11)
The uterine artery serves as a cost-effective and non-invasive tool for assessing the uteroplacental circulation which in turn reflects the placental implantation and trophoblastic invasion. The significance of uterine artery Doppler velocimetry is in the fact that it can predict the adverse effects associated with impaired placentation even when the disease process is inert, in the form of increased mean uterine artery PI. PI is the index most commonly used; its advantage over RI is that PI includes in its calculation the averaged value of all maximum velocities during the cardiac cycle, rather than just two points in the cardiac cycle. Uterine artery notching in the second trimester has a similar sensitivity to that of increased PI but there may be a degree of subjectivity in defining notching which further limits the value of this finding as a screening marker. Definite evidence has been available indicating the superiority of mean uterine artery PI as the preferred Doppler parameter for PE screening (12,13,14).
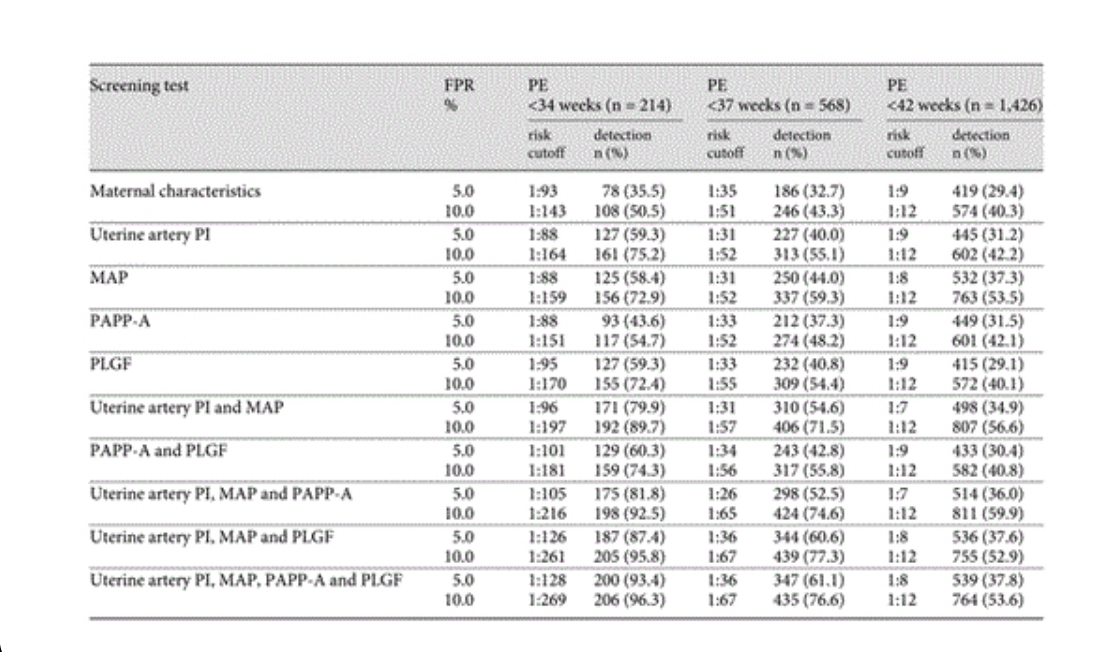
The detection rate of pre-eclampsia can be increased by>90 % when all the parameters are used in combination- MAP, Uterine artery PI, PAPPA, and PLGF at a false positive rate of 10%. (10)
The uterine artery Doppler PI is significantly increased in pregnant women at 11-13 weeks gestation (8) and is a better predictor of early onset rather than a late-onset disease, hence doing first-trimester uterine artery Doppler is justifiable, in the fact that it helps in early therapeutic interventions. UA mean PI significantly decreases within each of the two intervals at 11 to 13 weeks and 20 to 24 weeks and the persistence of an abnormal mean PI from the first to the second trimester identifies pregnant women with the greatest risk for PE and/or fetal growth restriction.
But when uterine artery PI is used in isolation its sensitivity in predicting preeclampsia and fetal growth restriction is moderate in low-risk pregnant women. Moreover, when combining the two biophysical markers MAP and Mean Uterine artery PI in calculating the patient-specific risk for PE, the correlation factors must be taken into consideration to avoid overestimating the contributions from each marker to provide an accurate risk assessment for PE.
Multiple Factors are known to affect uterine artery impedance including maternal heart rate, use of antihypertensives, hormonal changes in the menstrual cycle and chronic hyperandrogenism in the polycystic ovarian syndrome.
Unilateral location of the placenta is known to cause unilateral increased PI and carries a greater risk of PE and low birth weight than bilaterally normal PI, however, this effect appears to be eventually mediated through mean UtA PI z-score, which is relatively increased in these cases(15).
Variation is noted in the depiction of pre-eclampsia by mean uterine artery PI in twin pregnancies mainly due to decreased impedance to flow (affected by the larger placental size), with no differences related to chronicity. The relative increase of uterine artery PI found in twin pregnancies that developed early PE and SGA of both twins suggests that first-trimester uterine artery assessment may be useful in identifying such complications.
Note should also be made of significant difference in the ultrasound measurements of the uterine artery PI through two different routes transabdominal and transvaginal. The latter approach yielded significantly higher values than the first (17).
CONCLUSION
Uterine artery Doppler is a novel non-invasive imaging tool that helps in the prediction of pre-eclampsia by identifying high-risk women and offers an opportunity for early therapeutic interventions in the form of administering of low dose aspirin and also for follow-up of these women to prevent adverse maternal and perinatal outcome. Persistent abnormal mean uterine artery PI in both first and second-trimester intervals identified pregnant women with the greatest risk for PE and/or fetal growth restriction. But also a point to note that the predictive accuracy of first-trimester uterine artery Doppler is better in the detection of early-onset preeclampsia and FGR than late-onset disease. The uterine artery acts as a better predictor for preterm PE when used combined with other biophysical and biochemical parameters. The uterine artery when used in isolation has lower rates of detection thus limiting its utility as a disease marker in isolation. There also exists a significant difference in Mean PI values when measured via transabdominal and transvaginal routes. The uterine Doppler PI values were statistically significantly lower in twins than in singleton pregnancies.
References
- Wang W, Xie X, Yuan T, Wang Y, Zhao F, Zhou Z, et al. Epidemiological trends of maternal hypertensive disorders of pregnancy at the global, regional, and national levels: a population-based study. BMC Pregnancy Childbirth. 2021 May 8;21(1):364. doi: 10.1186/s12884-021-03809-2. PMID: 33964896; PMCID: PMC8106862.
- Steegers EA, von Dadelszen P, Duvekot JJ, Pijnenborg R. Pre-eclampsia. Lancet. 2010 Aug 21;376(9741):631-44. doi: 10.1016/S0140-6736(10)60279-6. Epub 2010 Jul 2. PMID: 20598363..
- Ives CW, Sinkey R, Rajapreyar I, Tita ATN, Oparil S. Preeclampsia-Pathophysiology and Clinical Presentations: JACC State-of-the-Art Review. J Am Coll Cardiol. 2020 Oct 6;76(14):1690-1702. doi: 10.1016/j.jacc.2020.08.014. PMID: 33004135.
- Bujold E, Roberge S, Lacasse Y, Bureau M, Audibert F, Marcoux S, et al: Prevention of preeclampsia and intrauterine growth restriction with aspirin started in early pregnancy: a meta-analysis. Obstet Gynecol 2010;116:402–414. https://doi.org/10.1097%2FAOG.0b013e3181e9322a
- Roberge S, Villa P, Nicolaides KH, Giguère Y, Vainio M, Bakthi A, et al. Early administration of low dose aspirin for the prevention of preterm and term pre- eclampsia: a systematic review and meta-analysis. Fetal Diagn Ther 2012;31:141–146. https://doi.org/10.1159%2F000336662
- Uzan J, Carbonnel M, Piconne O, Asmar R, Ayoubi JM. Pre-eclampsia: pathophysiology, diagnosis, and management. Vasc Health Risk Manag. 2011;7:467-74. doi: 10.2147/VHRM.S20181. Epub 2011 Jul 19. PMID: 21822394; PMCID: PMC3148420.
- Berry C, Atta MG. Hypertensive disorders in pregnancy. World J Nephrol. 2016 Sep 6;5(5):418-28. doi: 10.5527/wjn.v5.i5.418. PMID: 27648405; PMCID: PMC5011248.
- Wright D, Akolekar R, Syngelaki A, Poon LCY, Nicolaides KH: A competing risks model in early screening for preeclampsia. Fetal Diagn Ther 2012; E-pub ahead of print; DOI: 10.1159/000338470.
- Bujold E, Roberge S, Lacasse Y, Bureau M, Audibert F, Marcoux S, et al. Prevention of preeclampsia and intrauterine growth restriction with aspirin started in early pregnancy: a meta-analysis. Obstet Gynecol 2010;116:402–414.
- O’Gorman N, Wright D, Syngelaki A, Akolekar R, Wright A, Poon LC, Nicolaides KH. Competing risks model in screening for preeclampsia by maternal factors and biomarkers at 11-13 weeks’ gestation. Am J Obstet Gynecol 2016; 214: 103.e1-103.e12.
- Gallo DM, Wright D, Akolekar R, Poon LC, Nicolaides KH. Competing risks model in screening for preeclampsia by maternal factors and biomarkers at 19-24 weeks’ gestation. Am J Obstet Gynecol 2016; 214: 619.e1-619.e17.
- Lubis MP, Hariman H, Lumbanraja SN, Bachtiar A. The Role of Placental Growth Factor, Soluble Endoglin, and Uterine Artery Diastolic Notch to Predict the Early Onset of Preeclampsia. Open Access Maced J Med Sci. 2019 Apr 14;7(7):1153-1159. doi: 10.3889/oamjms.2019.154. PMID: 31049099; PMCID: PMC6490477.
- Rolnik DL, Wright D, Poon LCY, Syngelaki A, O’Gorman N, de Paco Matallana C, et al. ASPRE trial: performance of screening for preterm pre-eclampsia. Ultrasound Obstet Gynecol. 2017 Oct;50(4):492-495. doi: 10.1002/uog.18816. Epub 2017 Aug 24. Erratum in: Ultrasound Obstet Gynecol. 2017 Dec;50(6):807. PMID: 28741785.
- Tan MY, Wright D, Syngelaki A, Akolekar R, Cicero S, Janga D, et al. Comparison of diagnostic accuracy of early screening for pre-eclampsia by NICE guidelines and a method combining maternal factors and biomarkers: results of SPREE. Ultrasound Obstet Gynecol. 2018 Jun;51(6):743-750. doi: 10.1002/uog.19039. Epub 2018 Mar 14. PMID: 29536574.
- Dagklis T, Tsakiridis I, Zavlanos A, Athanasiadis A, Dinas K, Sotiriadis A. The effect of placental laterality at 20-24 gestational weeks on uterine artery doppler indices, fetal growth and preeclampsia. J Matern Fetal Neonatal Med. 2022 Jul;35(13):2493-2498. doi: 10.1080/14767058.2020.1786521. Epub 2020 Jul 13. PMID: 32660286.
- Rizzo, G., Pietrolucci, M.E., Aiello, E., Capponi, A. and Arduini, D. (2014), Uterine artery Doppler evaluation in twin pregnancies at 11 + 0 to 13 + 6 weeks of gestation. Ultrasound Obstet Gynecol, 44: 557-561. doi:10.1002/uog.13340
- Plasencia W, Barber MA, Alvarez EE, Segura J, Valle L, Garcia-Hernandez JA. Comparative study of transabdominal and transvaginal uterine artery Doppler pulsatility indices at 11-13 + 6 weeks. Hypertens Pregnancy. 2011;30(4):414-20. doi: 10.3109/10641955.2010.506232. Epub 2010 Dec 21. PMID: 21174578.
