1Raj Sonography & X- Ray Clinic, Baiju Choraha, Nayapura, Guna, Madhya Pradesh, India.
Short Title: Colour Doppler Study for FGR
*Corresponding Author: Lalit K Sharma, MD, Raj Sonography & X- Ray Clinic, Baiju Choraha, Nayapura, Guna, Madhya Pradesh, India E-mail : drlksharma_guna@yahoo.co.in
Keywords: Doppler Ultrasound, Fetal Growth Restriction, Perinatal Care
Established Facts and Novel Insights
- Established Facts
Prenatal identification of small-for-gestational age (SGA) babies results in a reduction of adverse perinatal outcomes and stillbirth.
Most instances of avoidable stillbirth are related with a failure to detect SGA in the antenatal period
- Novel Insights
Integrating colour Doppler studies with 3rd trimester ultrasound exams can help early identification of FGR. Doppler studies of the Cerebro-Placental ratio and mean Uterine Artery PI combined with estimates of fetal weight provide better risk estimates of FGR than isolated umbilical artery Doppler studies
Abstract
Introduction: Fetal growth restriction (FGR) and Small for Gestational Age (SGA) babies are major causes for preterm births and low birth weight, and consequently perinatal mortality in India. Integrating colour Doppler Studies with routine 3rd trimester ultrasound exams can help early identification of FGR and guide management
Case presentation:
We present two cases with the same gestational age (34+6 weeks) and almost similar estimated fetal weight percentiles (18th and 19th) respectively . All Doppler indices were normal for one fetus while the other fetus showed abnormal Doppler indices for mean Uterine artery PI, Middle Cerebral artery, Cerebro-Placental ratio and Umbilical artery PI. The fetus with abnormal Doppler indices was classified as Stage 1 FGR and recommended weekly follow ups while the other fetus with similar estimated fetal weight percentiles and normal Doppler was recommended follow up as per routine schedules.
Conclusion: The onset of Stage 1 FGR was picked up only by the integration of colour Doppler studies and will have been missed if only estimated fetal weight was considered.
Introduction
India has a high perinatal mortality rate.[1] Preterm births and low birth weight are major drivers of the high perinatal mortality rate in India. [1] Fetal growth restriction (FGR) is a major cause for preterm births and low birth weight in India and consequently perinatal mortality. [1]
Detection of FGR is important as these fetuses have poor perinatal outcomes in the short term including perinatal death, fetal distress and neurological injury and long term implications on health during adulthood. [2-7] Early identification and staging of FGR helps customize follow up schedules and appropriate planning regarding the timing and mode of childbirth and subsequent neonatal care. Colour Doppler studies have a very important role in the management of FGR. We will discuss the importance of each of these parameters in this case presentation.
Case Report/Case Presentation
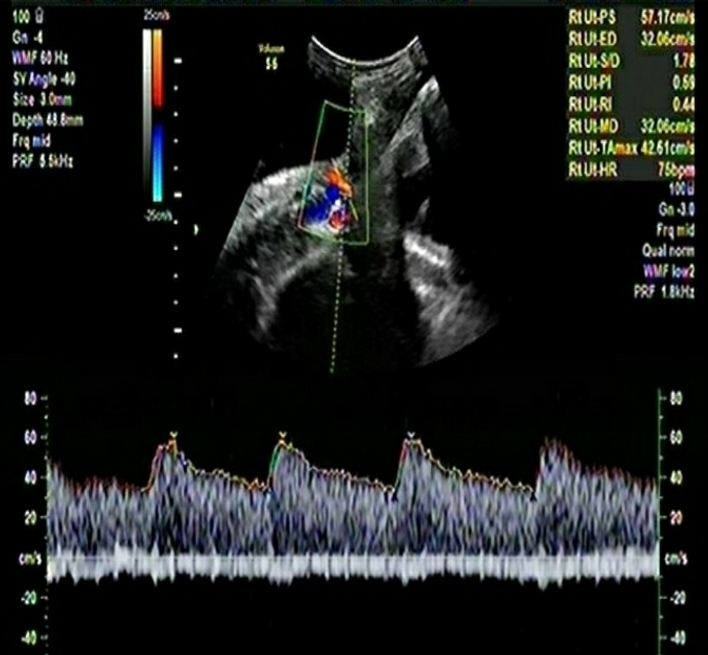
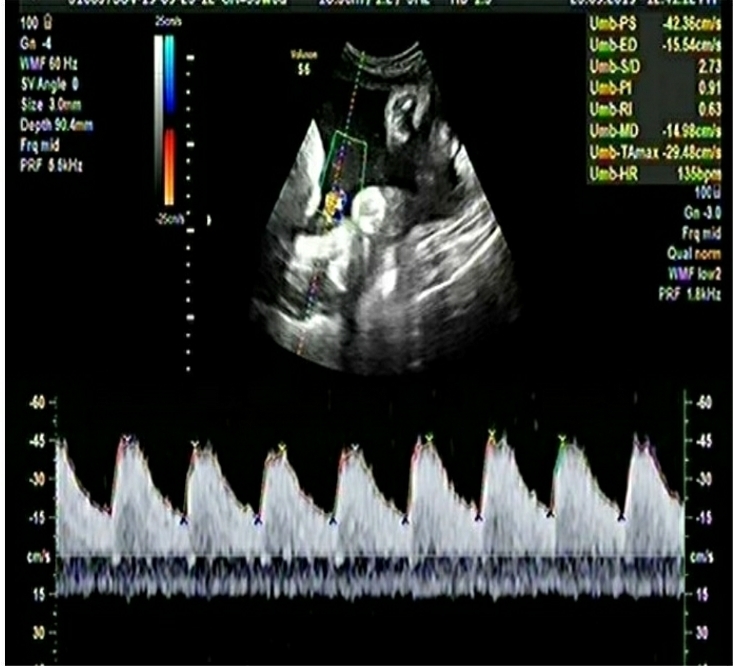
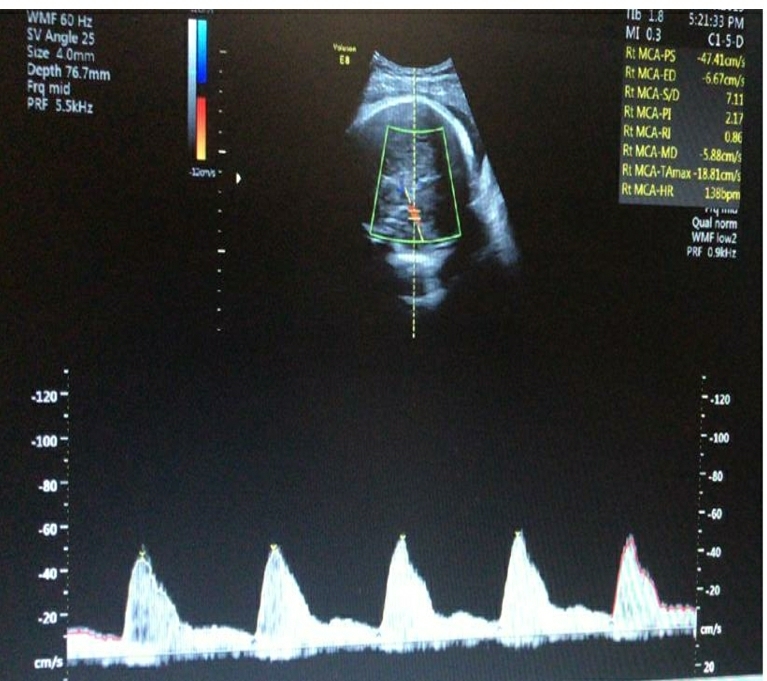
The important details of the two cases are summarized in Table-1.
Table-1: Comparison of important Doppler and clinical parameters of the two cases in the 3rd trimester
| Case 1 | Case 2 | |
| GA | 34 +6 weeks | 34+ 6 weeks |
| Mean Uterine Artery PI | 0.99 | 0.91 |
| Mean Uterine artery PI Centile | 69 | 50 |
| Middle Cerebral Artery PI | 1.20 | 1.47 |
| Middle Cerebral Artery Centile | 1 | 8 |
| Cerebro-placental Ratio | 1.21 | 1.61 |
| Cerebro-placental Ratio Centile | 1 | 17 |
| Umbilical Artery PI | 1.38 | 0.68 |
| Umbilical Artery Centile | 100 | 82 |
| Estimated Fetal Weight Centile | 19 | 18 |
| Inference | Stage 1 FGR | Normal fetus |
Discussion
This Case presentation aims to highlight several learning points and to discuss the importance integrating colour Doppler Studies with routine 3rd trimester ultrasound exams . The non integration of colour Doppler studies and reliance only on fetal biometry and estimated fetal weight will have led us to miss the diagnosis of Stage 1 FGR. This will have limited the opportunity to closely monitor progress and potential deterioration in this case.
Late-onset FGR represents the majority (70-80%) of FGR and has a low association with PE. Placental disease may be minimal or mild and the umbilical artery (UA) Doppler may be normal in almost all cases.[8]. However, these cases may show a high association with abnormal Cerebro-placental (CPR) values and Middle Cerebral Artery (MCA) PI<5, which suggests advanced brain vasodilation suggesting chronic hypoxia, may occur in 25% of late FGR [8]. Although advanced signs of fetal deterioration with changes in the DV are usually not visible in late onset FGR, there remains the possibility of acute fetal decline, fetal distress and neonatal acidosis.[9] Late FGR may be unpredictable in its course and can cause severe injury or death without observable late-stage signs as in early FGR possibly explained by a very low tolerance of term fetuses to hypoxia, the more frequent presence of uterine contractions at term, and even rapid placental function failure.[9]
Umbilical Artery Doppler
Traditionally, the umbilical artery (UA) Doppler studies have been relied on to consider a diagnosis of FGR. Meta analysis have provided evidence that UA Doppler can improve mortality rates and perinatal outcomes in FGR. [10] However, UA fails to identify mild placental disease that make up the majority of early-onset cases and almost all late-onset FGR. Additionally, SGA, considered to have a normal UA pulsatility index (PI), includes a significantly large proportion of fetuses with worse perinatal outcomes than normally grown fetuses. UA Doppler provides both diagnostic and prognostic information. The progression of UA Doppler patterns to absent or reverse end-diastolic flow correlates with the risks of injury or death. UA Doppler in high-risk pregnancies improves perinatal outcomes, with a 29% reduction (2-48%) in perinatal deaths [10]. Absent or reversed end-diastolic velocities maybe found on average 1 week before the acute deterioration and may be found in nearly 40% of fetuses with acidosis.[11]
Middle Cerebral Artery (MCA) Doppler
MCA provides information about vasodilation in the brain, which is a surrogate marker of hypoxia. MCA PI <5th centile is associated with adverse perinatal and neurological outcome and is useful for the identification [8] and prediction [12,13] of adverse outcome among late-onset FGR, independent of UA Doppler. The risk for emergency caesarean section for fetal distress is six times higher in fetuses with abnormal MCA PI compared with SGA fetuses. Late FGRs with abnormal MCA PI are also at higher risk for poorer neurobehavioral competence at birth and at 2 years of age [12,14].
Cerebroplacental Ratio (CPR)
The CPR is a diagnostic index that improves the sensitivity of UA and MCA as increased placental impedance (UA) is often combined with reduced cerebral resistance (MCA). The CPR may already be decreased when the UA or MCA show mild changes that are still within normal ranges [15,16]. Abnormal CPR is present in about 25% of late SGA fetuses before delivery and is associated with a higher risk of adverse outcome at induction. An abnormal CPR predicts neurobehavioral problems at 18 months of age [17]. The anterior cerebral artery-CPR rather than the MCA-CPR shows a stronger association, indicating a differential impact of regional alterations in cerebral blood flow impedance on development that is consistent with findings in early FGR [18,19].
Ductus Venosus (DV) Doppler
DV is a strong predictor of the short-term risk of fetal death in early-onset FGR. DV flow waveforms become abnormal only in advanced stages of fetal compromise [20] and there is a good correlation of abnormal DV waveform with late-stage acidemia [21]. Absent or reversed velocities are associated with perinatal mortality independent of GA at delivery [22], with a risk ranging from 40 to 100% in early-onset FGR [23]. An abnormal DV is an indication to recommend delivery at any GA after completion of steroids. A DV above the 95% centile is associated with higher risks but not as consistently as when atrial flow is reverse. Abnormal DV precedes the loss of short-term variability (STV) in computerized cardiotocography (cCTG) in a about 50% of cases and is abnormal 48-72 h before the biophysical profile (BPP) in about 90% of cases [20]. Thus, abnormal DV provides a better indication for delivering fetuses in critical conditions at very early gestational ages.
Aortic Isthmus Doppler
The aortic isthmus (AoI) Doppler reflects there relation between brain impedance and systemic vascular systems and is associated with increased fetal mortality and neurological morbidity in early-onset FGR [24]. Reverse AoI flow indicates advanced deterioration.
There is no evidence to support the use of traditional fetal heart rate (FHR) monitoring or ‘non-stress tests’ in FGR fetuses and false positive rates are very high. A main limitation of conventional CTG is the subjective interpretation of the FHR. cCTG evaluates STV of the FHR, an aspect that subjective evaluation cannot assess and is sensitive to detect advanced fetal deterioration providing a value similar to DV reverse atrial flow for the short-term prediction of fetal death. Biophysical Profile(BPP) is calculated by combining ultrasound assessment of fetal tone, respiratory and body movements, with amniotic fluid index and a conventional CTG. However, a high false-positive rate (50%) limits the clinical usefulness of the BPP [25]. A meta-analysis [26] showed no significant benefit of BPP in high-risk pregnancies. Consequently, whenever Doppler expertise and/or cCTG are available, the incorporation of BPP in management protocols of FGR is questionable.
Currently, the best test to
differentiate FGR from SGA is the Doppler cerebroplacental ratio (CPR). CPR reflects
even mild increases in placental resistance with mild reductions in fetal brain
vascular resistance and correlates better with adverse outcome [14]. The
uterine artery Doppler PI (UtA PI) can be abnormal even if UA Doppler is normal
and predicts a poorer outcome in small fetuses. A very small EFW <3rd
percentile is another predictor of poor outcome. Fetuses with an EFW <3rd
percentile have a much higher risk of
adverse perinatal outcome irrespective of the CPR and UtA Doppler indices [27].
The risk of adverse perinatal outcomes is increased when any one of CPR, UtA PI
or EFW <3rd percentile is abnormal. A recent study reported that
the risk of caesarean section for fetal distress or neonatal acidosis was 8% in
controls, 11% when all three parameters CPR,
UtA PI or EFW <p3 were normal and 36% when any one if these was abnormal [28].
References
- International Institute for Population Sciences (IIPS) and ICF. 2017. National Family Health Survey (NFHS-4) 2015-16: India. Mumbai: IIPS
- Lindqvist PG, Molin J: Does antenatal identification of small-for-gestational age fetuses significantly improve their outcome? Ultrasound Obstet Gynecol 2005;25:258-264.
- Gardosi J, et al: Maternal and fetal risk factors for stillbirth: population-based study. BMJ 2013;346:f108.
- Richardus JH, Graafmans WC, Verloove-Vanhorick SP, Mackenbach JP; EuroNatal International Audit Panel; EuroNatal Working Group. Differences in perinatal mortality and suboptimal care between 10 European regions: results of an international audit. BJOG. 2003 Feb;110(2):97-105.
- Larroque B, Bertrais S, Czernichow P, Léger J. School difficulties in 20-year-olds who were born small for gestational age at term in a regional cohort study. Pediatrics. 2001 Jul;108(1):111-5.
- Crispi F, Bijnens B, Figueras F, Bartrons J, Eixarch E, Le Noble F, Ahmed A, Gratacós E. Fetal growth restriction results in remodeled and less efficient hearts in children. Circulation. 2010 Jun 8;121(22):2427-36. doi: 10.1161/CIRCULATIONAHA.110.937995.
- Verkauskiene R, Figueras F, Deghmoun S, Chevenne D, Gardosi J, Levy-Marchal M. Birth weight and long-term metabolic outcomes: does the definition of smallness matter? Horm Res. 2008;70(5):309-15.
- Oros D, Figueras F, Cruz-Martinez R, Meler E, Munmany M, Gratacos E. Longitudinal changes in uterine, umbilical and fetal cerebral Doppler indices in late-onset small-for-gestational age fetuses. Ultrasound Obstet Gynecol. 2011 Feb;37(2):191-5.
- Figueras F, Eixarch E, Meler E, Iraola A, Figueras J, Puerto B, Gratacos E. Small-for-gestational-age fetuses with normal umbilical artery Doppler have suboptimal perinatal and neurodevelopmental outcome. Eur J Obstet Gynecol Reprod Biol. 2008 Jan;136(1):34-8.
- Alfirevic Z, Stampalija T, Gyte GM: Fetal and umbilical Doppler ultrasound in high-risk pregnancies. Cochrane Database Syst Rev 2010:CD007529.
- Ferrazzi E, Bozzo M, Rigano S, Bellotti M, Morabito A, Pardi G, Battaglia FC, Galan HL. Temporal sequence of abnormal Doppler changes in the peripheral and central circulatory systems of the severely growth-restricted fetus. Ultrasound Obstet Gynecol. 2002 Feb;19(2):140-6.
- Eixarch E, Meler E, Iraola A, Illa M, Crispi F, Hernandez-Andrade E, Gratacos E, Figueras F. Neurodevelopmental outcome in 2-year-old infants who were small-for-gestational age term fetuses with cerebral blood flow redistribution. Ultrasound Obstet Gynecol. 2008 Dec;32(7):894-9.
- Hershkovitz R, Kingdom JC, Geary M, Rodeck CH. Fetal cerebral blood flow redistribution in late gestation: identification of compromise in small fetuses with normal umbilical artery Doppler. Ultrasound Obstet Gynecol. 2000 Mar;15(3):209-12.
- Oros D, Figueras F, Cruz-Martinez R, Padilla N, Meler E, Hernandez-Andrade E, Gratacos E. Middle versus anterior cerebral artery Doppler for the prediction of perinatal outcome and neonatal neurobehavior in term small-for-gestational-age fetuses with normal umbilical artery Doppler. Ultrasound Obstet Gynecol. 2010 Apr;35(4):456-61.
- Gramellini D, Folli MC, Raboni S, Vadora E, Merialdi A. Cerebral-umbilical Doppler ratio as a predictor of adverse perinatal outcome. Obstet Gynecol. 1992 Mar;79(3):416-20.
- Arbeille P, Maulik D, Fignon A, Stale H, Berson M, Bodard S, Locatelli A. Assessment of the fetal PO2 changes by cerebral and umbilical Doppler on lamb fetuses during acute hypoxia. Ultrasound Med Biol. 1995;21(7):861-70.
- Roza SJ, Steegers EA, Verburg BO, Jaddoe VW, Moll HA, Hofman A, Verhulst FC, Tiemeier H. What is spared by fetal brain-sparing? Fetal circulatory redistribution and behavioral problems in the general population. Am J Epidemiol. 2008 Nov 15;168(10):1145-52.
- Figueroa-Diesel H, Hernandez-Andrade E, Acosta-Rojas R, Cabero L, Gratacos E. Doppler changes in the main fetal brain arteries at different stages of hemodynamic adaptation in severe intrauterine growth restriction. Ultrasound Obstet Gynecol. 2007 Sep;30(3):297-302. .
- Dubiel M, Gunnarsson GO, Gudmundsson S: Blood redistribution in the fetal brain during chronic hypoxia. Ultrasound Obstet Gynecol 2002;20:117-121.
- Baschat AA, Gembruch U, Harman CR: The sequence of changes in Doppler and biophysical parameters as severe fetal growth restriction worsens. Ultrasound Obstet Gynecol 2001;18:571-577.
- Hecher K, Snijders R, Campbell S, Nicolaides K. Fetal venous, intracardiac, and arterial blood flow measurements in intrauterine growth retardation: relationship with fetal blood gases. Am J Obstet Gynecol. 1995 Jul;173(1):10-5.
- Schwarze A, Gembruch U, Krapp M, Katalinic A, Germer U, Axt-Fliedner R. Qualitative venous Doppler flow waveform analysis in preterm intrauterine growth-restricted fetuses with ARED flow in the umbilical artery–correlation with short-term outcome. Ultrasound Obstet Gynecol. 2005 Jun;25(6):573-9.
- Baschat AA, Gembruch U, Weiner CP, Harman CR. Qualitative venous Doppler waveform analysis improves prediction of critical perinatal outcomes in premature growth-restricted fetuses. Ultrasound Obstet Gynecol. 2003 Sep;22(3):240-5.
- Fouron JC, Gosselin J, Raboisson MJ, Lamoureux J, Tison CA, Fouron C, Hudon L. The relationship between an aortic isthmus blood flow velocity index and the postnatal neurodevelopmental status of fetuses with placental circulatory insufficiency. Am J Obstet Gynecol. 2005 Feb;192(2):497-503.
- Miller DA, Rabello YA, Paul RH: The modified biophysical profile: antepartum testing in the 1990s. Am J Obstet Gynecol 1996;174:812-817.
- Alfirevic Z, Neilson JP: Biophysical profile for fetal assessment in high risk pregnancies. Cochrane Database Syst Rev 2000:CD000038.
- Savchev S, Figueras F, Cruz-Martinez R, Illa M, Botet F, Gratacos E. Estimated weight centile as a predictor of perinatal outcome in small-for-gestational-age pregnancies with normal fetal and maternal Doppler indices. Ultrasound Obstet Gynecol. 2012 Mar;39(3):299-303.
- Figueras F, Savchev S, Triunfo S, Crovetto F, Gratacos E. An integrated model with classification criteria to predict small-for-gestational-age fetuses at risk of adverse perinatal outcome. Ultrasound Obstet Gynecol. 2015 Mar;45(3):279-85.