1 Dr. Shweta Nagar’s Ultrasound clinic & Imaging Centre, Flat no. 101, Princes Center, above Mama Loca cafe , 6/3 New Palasia. Indore.MP-452001
Short Title: Low Dose Aspirin in Pre-eclampsia
*Corresponding Author
Dr. Shweta Nagar, Dr. Shweta Nagar’s Ultrasound clinic & Imaging Centre, Flat no. 101, Princes Center, above Mama Loca cafe , 6/3 New Palasia. Indore.MP-452001. E-mail: shwetarnagar@gmail.com
Keywords:
Pre-eclampsia, Low dose Aspirin, Doppler Ultrasound, Pregnancy.
Established Fact
- Low Dose Aspirin, started between 11-14+6 weeks of gestation is effective in the prevention of preterm pre-eclampsia in pregnant women
Insights
- Maximum benefits occur when Low dose Aspirin is started between 11-14+6 gestation weeks, however, Low dose Aspirin may be beneficial to prevent onset of preterm pre-eclampsia in high risk pregnant women when started before 16 gestation weeks
- Effectiveness of low dose Aspirin reduces if started after 16 gestation weeks and has minimal or no effect if started after 20 gestation weeks
- Current evidence indicates optimal benefits from a dose of 150mg of aspirin given once daily at bedtime
Abstract
Introduction: There Is evidence that Low dose Aspirin, 150mg given once daily at bed time is effective to prevent onset of preterm pre-eclampsia in high risk pregnant women.
Case Presentation: A multiparous pregnant woman with a provisional diagnosis of hypertension and a history of preterm delivery in the previous pregnancy was referred for a routine ultrasound exam by the obstetrician. The woman presented at 16 gestation weeks and was determined to be at high risk for the development of preterm pre-eclampsia (PE). She was started on low dose aspirin 150 mg once daily at bedtime. She was advised follow up Doppler and ultrasound exams in therefore and third trimester but returned only at 37.5 gestation weeks for a routine growth scan. At 37.5 gestation weeks, she had not developed PE, had normal Doppler study and an estimated fetal weight (EFW) of 3020 grams.
Discussion/Conclusion: Low dose Aspirin at 150mg once daily at bed time, started early in pregnancy, may prevent onset of preterm PE in pregnant women at high risk for preterm PE. Although the optimal period to start low dose aspirin is 11-13+6 weeks, starting low dose aspirin before 20 gestation weeks may still provide benefits.
Introduction
The maternal mortality ratio in India has declined steadily and reported as 130 per 100,000 live births in 2014-16.[1] Pregnancy induced hypertension remains a major cause for maternal mortality, preterm deliveries, fetal growth restriction and low birth weight in India. [1] A large clinical trial, the ASPRE trial, has provided evidence that starting low dose aspirin at 150 mg per day from 11–14 weeks until 36 weeks’ gestation, in pregnant women identified as at high risk for preterm PE, reduces the incidence of preterm PE by > 60%.[2]
Case Report/Case Presentation
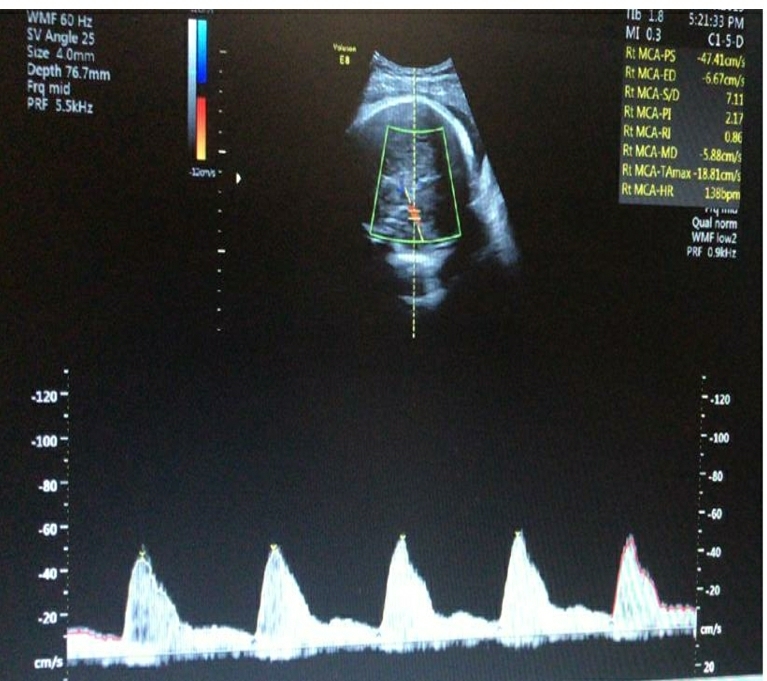
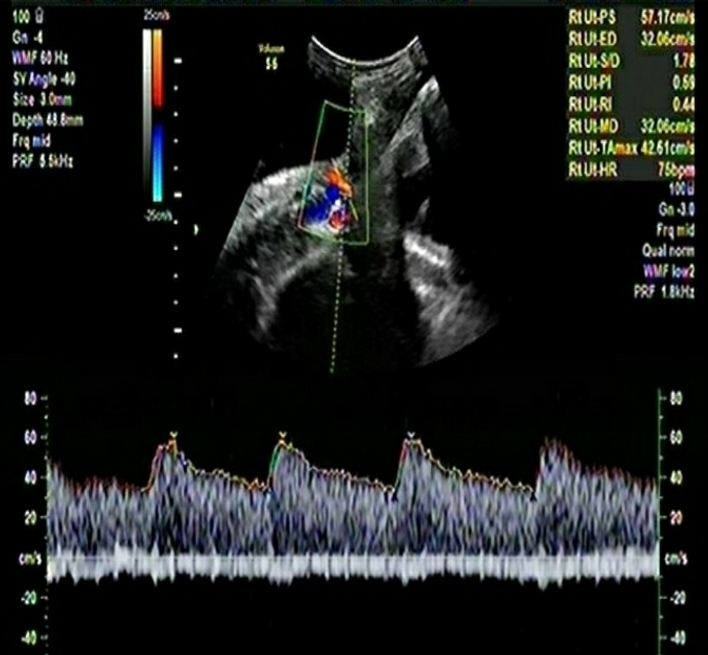
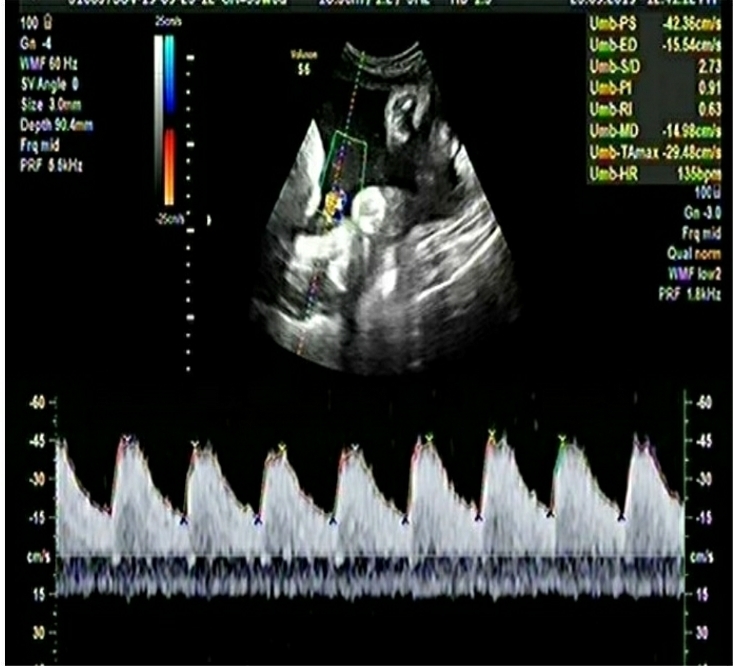
A multiparous woman presented for routine fetal growth ultrasound scan at 37.5 gestation weeks. On examination, she had mean uterine artery Doppler PI <95th centile, umbilical artery Doppler PI <95th centile, and Middle cerebral artery and Cerebro-Placental ratio >5th centile. The Amniotic Fluid Index was 11. Fetal biometry and growth parameters were within normal ranges and the estimated fetal weight was 3020gms.
The woman had initially presented to us at 16 weeks of gestation for a routine ultrasound exam. The referring obstetrician had diagnosed hypertension. The woman had a history of preterm delivery (31-32 gestation weeks) in the previous pregnancy. The cause of preterm birth in the previous pregnancy could not be verified with medical records. The woman was screened using a combined screening protocol [3] that incorporated demographic details like age, parity, height and weight of the woman, family history of PE, inter-pregnancy interval, use of assisted reproductive technology, comorbid conditions in pregnancy especially diabetes mellitus, chronic hypertension, systemic lupus erythematosus and anti-phospholipid syndrome, two readings of the systolic and diastolic blood pressure in both arms and determination of the mean arterial pressure using validated digital instruments and a standardized protocol, Ultrasound scan for dating of pregnancy and fetal biometry through a transabdominal approach, and Colour Doppler study of the right and left uterine arteries and determination of the mean uterine artery PI. A sagittal section of the uterus was obtained and the cervical canal and internal cervical os were identified. Subsequently, the transducer was gently tilted to the side in the midline and colour flow mapping was used to identify each uterine artery along the side of the cervix and uterus at the level of the internal os. Pulsed‐wave Doppler was used with the sampling gate set at 2 mm to cover the whole vessel and care was taken to ensure that the angle of insonation was less than 30°. When three similar consecutive waveforms were obtained, the Uterine artery PI was measured and the mean Uterine Artery PI of the left and right arteries was calculated. Biochemical markers were not assessed.
The woman was determined to be at high risk for the development preterm PE based on a combined screening protocol algorithm [3] that is used globally to determine risk estimates for preterm PE based on a priori risk factors and based on a 1 in 150 cutoff. The risk calculator is available free of charge at https://fetalmedicine.org/research/assess/preeclampsia.
Consistent with current evidence based recommendations for the management of pregnant women at high risk for preterm PE, the woman was advised low dose aspirin at 150mg once daily at bedtime to be continued till 36 gestation weeks. She was advised further follow up for a targeted fetal anomaly scan and further assessment of the risk for preterm PE at 20 gestation weeks and subsequent follow up exams at 4 week intervals. However, she did not return for follow up ultrasound exams as advised.
The target condition was PE based on the definition of the International Society for the Study of Hypertension in Pregnancy. [4] PE is defined as systolic blood pressure at ≥140 mm Hg and/or diastolic blood pressure at ≥90 mm Hg on at least two occasions measured 4 hours apart in previously normotensive women and is accompanied by one or more of the following new‐onset conditions at or after 20 weeks of gestation: Proteinuria (i.e. ≥30 mg/mol protein: creatinine ratio; ≥300 mg/24 hour; or ≥2 + dipstick);Evidence of other maternal organ dysfunction, including: acute kidney injury (creatinine ≥90 μmol/L; 1 mg/dL); liver involvement (elevated transaminases, e.g. alanine aminotransferase or aspartate aminotransferase >40 IU/L) with or without right upper quadrant or epigastric abdominal pain; neurological complications (e.g. eclampsia, altered mental status, blindness, stroke, clonus, severe headaches, and persistent visual scotomata); or haematological complications (thrombocytopenia–platelet count <150 000/μL, disseminated intravascular coagulation, haemolysis); or Uteroplacental dysfunction (such as fetal growth restriction, abnormal umbilical artery Doppler waveform analysis, or stillbirth).
Discussion
This case presentation highlights the potential benefits of starting low dose aspirin early in pregnant women at risk for the development of preterm PE. The case highlights the use of a combined screening protocol to estimate the risk for preterm PE in pregnant women. On assessment at term (37+ gestation weeks) in the third trimester, the woman had a normally growing fetus and had not developed preterm PE after starting low dose aspirin at 16 weeks and continuing till 36 gestation weeks.
PE occurs in up to 8% of pregnancies, is multifactorial in origin and maybe categorized as Preterm PE (with delivery at <37+0 weeks of gestation) and term PE (with delivery occurring after 37 gestation weeks). [5] The aetiology of PE differs between preterm and term PE although a certain amount of overlap may occur. Preterm PE is primarily associated with impaired presentation, placental vascular lesions and impaired or incomplete transformation of uterine spiral arteries while term PE is primarily associated with maternal factors.[5]
Conventionally, the identification of women at high risk of PE who may benefit from the prophylactic use of aspirin is based on maternal characteristics and medical history. The National Institute for Health and Care Excellence (NICE) recommends the identification of the high‐risk group on the basis of 10 factors, including maternal characteristics and features of the medical and obstetric histories. [6] However, screening using the NICE protocol is suboptimal with a detection rate (DR) of preterm PE of 39% at a FPR of 10%. [6] The American College of Obstetricians and Gynecologists (ACOG) recommends the use of aspirin for women with a history of PE in previous pregnancies or a history of PE that resulted in delivery before 34 weeks’ gestation. [7] However, this covers only a small subgroup of women (up to 5%) who may develop preterm PE. [8] The recent ASPRE trial provided evidence that women with singleton pregnancy identified by means of first‐trimester combined screening as being at high risk for preterm PE, had a reduction of preterm PE by >60% when administered aspirin at a dose of 150 mg per day from 11–14 weeks until 36 weeks’ gestation. [2]
One hypothesis for the development of PE is the deficient production of prostacyclin and an increased production of thromboxane A2 (TXA2) by placenta and platelets. This selective inhibition of cyclooxygenase and the resulting alteration in the prostacyclin to thromboxane A2 ratio in the placenta forms the basis of using aspirin to prevent or delay the onset of PE. [9,10]
Several guidelines exist for the administration of low dose aspirin. The World Health Organization recommends low dose aspirin (75 mg) before 20 weeks of pregnancy among women at high risk for PE. [11] The United States Preventive Task Force recommendation statement and ACOG recommend a low dose of aspirin (81 mg) prophylaxis from 12 weeks onwards. [7] However, the recent ASPRE trial showed a significant reduction in incidence of preterm PE (OR: 0.38, 95% CI: 0.20–0.74; p = 0.004) with a low dose of aspirin at 150mg once daily. [2]
The ASPRE trial has led to the evidence based recommendation that women identified at high risk after the first‐trimester screening and assessment for preterm PE should receive aspirin prophylaxis commencing at 11–14+6 weeks of gestation at a dose of 150 mg to be taken every night until either 36 weeks of gestation, when delivery occurs, or when PE is diagnosed. [2] There is also evidence that adverse events are lower when aspirin is taken at night compared to morning or afternoon.[12] There is evidence that only aspirin started before 16 gestation weeks or at doses >100mg per day is associated with optimal reduction of preterm PE. [13]
References
- Niti Ayog, India. Maternal Mortality Ratio (per 100,000 live births). Accessed online from https://www.niti.gov.in/content/maternal-mortality-ratio-mmr-100000-live-births , on Oct 24, 2019
- Rolnik DL, Wright D, Poon LC, O’Gorman N, Syngelaki A, de Paco Matallana C, et al. Aspirin versus placebo in pregnancies at high risk for preterm preeclampsia. N Engl J Med 2017; 377: 613– 622.
- Rolnik DL, Wright D, Poon LCY, et al. ASPRE trial: Performance of screening for preterm pre‐eclampsia. Ultrasound Obstet Gynecol. 2017; 50: 492– 495.
- Brown MA, Lindheimer MD, de Swiet M, Van Assche A, Moutquin JM. The classification and diagnosis of the hypertensive disorders of pregnancy: Statement from the international society for the study of hypertension in pregnancy (ISSHP). Hypertens Pregnancy 2001; 20: IX– XIV.
- FIGO Working Group on Good Clinical Practice in Maternal–Fetal Medicine. Good clinical practice advice: First trimester screening and prevention of pre‐eclampsia in singleton pregnancy. Int J Gynecol Obstet. 2019; 144: 325– 329.
- National Collaborating Centre for Women’s and Children’s Health (UK).Hypertension in pregnancy: the management of hypertensive disorders during pregnancy.London: RCOG Press, 2010.
- Hypertension in pregnancy: report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet Gynecol 2013; 122: 1122– 31.
- O’ Gorman N, Wright D, Poon LC, Rolnik DL, Syngelaki A, de Alvarado M1, et al. Multicenter screening for pre‐eclampsia by maternal factors and biomarkers at 11–13 weeks’ gestation: comparison with NICE guidelines and ACOG recommendations. Ultrasound Obstet Gynecol 2017; 49: 756– 760.
- Sibai BM. Thrombophilia and severe preeclampsia: Time to screen and treat in future pregnancies? Hypertension. 2005; 46: 1252– 1253.
- Dekker G, Sibai B. Primary, secondary, and tertiary prevention of pre-eclampsia. Lancet.2001; 35 : 209– 215.
- WHO. Recommendations for Prevention and Treatment of Pre-eclampsia and Eclampsia. Geneva. World Health Organization. 2011
- Ayala DE, Ucieda R, Hermida RC. Chronotherapy with low‐dose aspirin for prevention of complications in pregnancy. Chronobiol Int. 2013; 30: 260– 279.
- Roberge S, Bujold E, Nicolaides KH. Aspirin for the prevention of preterm and term preeclampsia: Systematic review and meta analysis. Am J Obstet Gynecol. 2018; 218: 287– 293.e1