Author: Dr. Shweta Nagar, Dr. Shweta Nagar’s Ultrasound clinic & Imaging Centre, Flat no. 101, Princes Center, above Mama Loca cafe , 6/3 New Palasia. Indore.MP-452001. E-mail: shwetarnagar@gmail.com
Purpose of the Guidelines
These guidelines are prepared on behalf of Samrakshan, a national program of Indian Radiological and Imaging Association (IRIA), to provide a roadmap for Radiologists in rural and semi urban India on the integration of Fetal Echocardiography with routine antenatal obstetric scans. These guidelines are intended for the use by fetal radiologists to encourage clinical practice-based teaching and research in diagnostic imaging pertinent to the screening and confirmation of fetal cardiac diseases in the antenatal period. The aim of these Guidelines is to provide evidence‐based recommendations regarding the role of Fetal Echocardiography. The primary focus of these guidelines is on the technical/clinical aspects of Fetal Echocardiography. These guidelines do not intend to establish a legal standard of care, as the evidence that underpins the development of these guidelines are dynamic and maybe influenced by individual circumstances, local protocol and available resources and changes in the evidence.
Congenital Heart Disease: The Problem
Congenital heart disease (CHD) is one of the most common congenital disorders and is responsible for 28% of all congenital birth defects [1]. The birth prevalence of CHD is reported to be 8-12/1000 live births globally [2,3] with nearly 1.35 million babies born with CHD each year globally [4]. The birth prevalence of severe CHD is approximately 1.5 – 1.7/1000 live births [3,6,7]. The use of fetal echocardiography has helped to identify more milder cases resulting in a higher birth prevalence [6-8].
Over 200,000 children are estimated to be born with congenital heart disease in India every year.[8] The true incidence or birth prevalence has been reported only in few studies from India and have primarily included only babies born in a hospital setting [9-12]. Vaidyanathan, et al, reported a birth prevalence for minor CHD of 74.4/1000 live births and 3.1/1000 live births for major CHD based on echocardiography for all newborns [10]. A CHD likely to normalize by 6 weeks was categorized as a minor CHD and a CHD likely to require early intervention was categorized as a major CHD. Saxena, et al, [12] reported a birth prevalence of 8.1/1000 live births (95% CI 6.94; 9.40). Significant CHD was defined as atrial septal defect >5 mm, patent ductus arteriosus >2 mm with left ventricle volume overload, restrictive VSD, and valvular aortic/pulmonary stenosis with gradients <25 mmHg (in addition to Major CHD). Sawant, et al, reported a birth prevalence of 13.3/1000 live births but had done echocardiography only for suspected cases [11]. Khalil, et al, reported a birth prevalence of 3.9/1000 live births, however, this study did not include echocardiography and was based only on clinical examination [9].
How does Antenatal Screening for cardiac diseases help?
Fetal echocardiography can provide prognostic information that helps for a smooth transition between the pre- and post-natal states and the opportunity to provide immediate care at birth, thereby avoiding the onset of hemodynamic compromise. Recent studies suggest improved physiological state after birth and improved surgical outcome for infants who have had prenatal diagnosis via fetal echocardiography.
In addition, accurate diagnosis via fetal echocardiography allows for appropriate counselling and gives parents the opportunity to learn about the cardiac anomaly. This knowledge can allay parental fears, improve psychological state and bolster coping skills for parents to deal with the birth of a child with life-threatening cardiovascular illness.
Fetal Echocardiography can help to determine various treatment options before and after delivery. It can help to decide to continue or terminate the pregnancy or offer intrauterine interventions. It is mandatory to plan for childbirth at a tertiary level care centre to improve neonatal and perinatal outcomes.
Who can Screen for cardiac diseases in the antenatal period?
Screening in prenatal care has been commonly used to offer the option of timely termination of pregnancy to parents of fetuses with untreatable conditions. A general antenatal obstetrical ultrasound can function as a screen for fetal cardiovascular disorders. Suspicion or detection of a fetal cardiovascular abnormality requires referral for a more comprehensive evaluation to a physician with expertise in fetal echocardiography.
All radiologists should perform screening of heart during all routine obstetrical ultrasound studies done beyond 18 weeks of gestational age.
Fetal Echocardiography can be done by well-trained fetal radiologists, paediatric cardiologists, maternal-fetal medicine specialists and those who have acquired the appropriate knowledge base and skills as outlined below
- Be knowledgeable in the principles of biological ultrasound instrumentation and its application in human pregnancy
- Have a thorough understanding of maternal-fetal physiology as well as maternal conditions that may affect the developing fetus
- Have knowledge of the anatomy and physiology of the cardiovascular system throughout the stages of human development
- Have the skill and ability to apply all modalities of echocardiography including 2-dimensional, M-mode, pulsed-wave, continuous wave, and Doppler color flow mapping in recognizing and evaluating both the normal and abnormal fetal cardiovascular state
- Be able to recognize the full spectrum of simple and complex, acquired and congenital, heart disease and its manifestations and natural history throughout gestation, and recognize the limitations of fetal echocardiography in detecting important associated lesions
- Have a thorough understanding of the spectrum of fetal arrhythmias and the ability to utilize the spectrum of echocardiographic modalities for their assessment
- Be familiar with the latest developments in obstetrical diagnostics, which include invasive and non-invasive tests available throughout pregnancy
- Have knowledge of the growing field of invasive fetal intervention and its possible effects on the fetal cardiovascular system
Where can antenatal screening for cardiac diseases be done?
A primary screen for cardiac diseases should be performed by all radiologists as part the antenatal screening. Confirmatory Echocardiography may be done at secondary and tertiary care levels with access to consultative services.
Fetal radiologists performing a confirmatory echocardiography should have access to a multidisciplinary team with expertise in maternal-fetal medicine, genetics, neonatology, paediatric surgery, paediatric cardiology, and paediatric cardiac surgery, with availability for consultative services and advice.
Common indications for Fetal Echocardiography
Maternal Indications
- Family history of a sibling or any parent with history of CHD
- First- or second-degree relative with disease of Mendelian inheritance and a history of childhood cardiac manifestations.
- Second-degree relative of a fetus with CHD
- High risk pregnancy/IVF/Multiple pregnancy
- Exposure to prostaglandin synthetase inhibitors (eg, ibuprofen, salicylic acid, indomethacin)
- Rubella infection
- Autoimmune disease (eg, SLE, Sjögren’s)
- Familial inherited disorders (Ellisvan Creveld, Marfan’s, Noonan’s, etc)
- Teratogens/maternal DM /PKU
- Pregestational diabetes regardless of the hemoglobin A1C level.
Fetal Indications
- Abnormal NT scan findings like increased nuchal transluceny /absent nasal bone /abnormal ductus venosus waveform &/or tricuspid regurgitation.
- Nuchal translucency of 3.5 mm or greater or at or above the 99th percentile for gestational age.
- Abnormal 2nd trimester ultrasound screening
- Chromosomal /extracardiac abnormality
- Past h/o Fetal hydrops
- Persistent fetal tachycardia (heart rate > 180 beats per minute)
- Persistent fetal bradycardia (heart rate < 120 beats per minute) or a suspected heart block
- Frequent episodes or a persistently irregular cardiac rhythm
- Oligoamnios /Polyhydramnios.
Other considerations
- Obesity (body mass index ≥30 kg/m2)
- Noncardiac “soft marker” for aneuploidy in the absence of karyotype information.
- Abnormal maternal serum analytes (eg, α-fetoprotein level)
- Isolated single umbilical artery.
When should the test be done?
Early Fetal cardiac screening can be performed at 11-13 weeks gestation to look for
- Colour flow in the ductus venosus and colour sampling and spectral waveform
- To look for normal right atrioventricular flow in apical four chamber view on colour Doppler
- Tricuspid regurgitation.
Any abnormality detected at 11-13 weeks can be a strong marker for cardiac anomaly or chromosomal anomaly and these subjects need further chromosomal screening and dedicated fetal echocardiography in the 2nd trimester.
The optimal timing for performance of a comprehensive trans abdominal fetal echocardiogram is between 18 to 22 weeks gestation.
Acquiring images of the fetal heart at 15 to 18 weeks is possible. However, performing a comprehensive cardiac evaluation study at this age can be difficult and may require repeat assessment at 18 to 22 weeks.
Basic equipment
Ultrasound systems used for fetal echocardiography should have capabilities for performing excellent B-mode -2-Dimensional, M-mode, and Doppler imaging and good cine acquisition.
The equipment used for performance of fetal echocardiography also needs a good cine-loop facility so that one can scroll back frame by frame and capture the frame of interest. The system should have colour Doppler, pulsed Doppler, and continuous wave Doppler. STIC, tissue Doppler, and multiplanar imaging are added advantages.
A special pre-set for fetal heart evaluation needs to be created with a high frame rate of 80 to 100 Hz to view important events occurring at heart rates in excess of 140 beats per minute, minimized persistence, and increased compression. The system should have the ability to zoom the image without causing deterioration of image quality. A higher pulse repetition frequency (PRF) is required for colour Doppler in the fetus as compared to the settings used for routine obstetric colour Doppler. Doppler including colour, pulse, high pulse repetition frequency, and continuous wave should be available.
Tissue Doppler imaging has been recently applied in the assessment of fetal arrhythmia. Harmonic imaging is useful when acoustic penetration is difficult such as in the presence of maternal obesity. Phased array transducers with fundamental frequencies between 4 and 12 MHz are generally used. Curvilinear trans abdominal probes may be helpful given the wider near-field of view. High frequency transducers with a narrower footprint commonly used in echocardiography of infants may also be helpful.
The degree of image magnification should be adjusted so that the heart fills about one-third of the imaging sector display. Excellent zoom facility without image distortion or any compromise on the image resolution is essential.
Newer USG systems have dedicated 3D, 4D, volume software like HD eSTIC COLOUR FLOW, 4D VALVES, TUI (Tomographic ultrasound imaging), VCAD (Volume Computer Aided Diagnosis) for multiplanar imaging.
Retest
In some cases, it may be necessary to reexamine the patient at a different time or different sittings during gestation if the heart is poorly visualized due to technical factors.
Basic Technique
- Sliding laterally. This can, for example, move the thorax into the centre of the scan plane
- Rocking from side-to-side (laterally). This can produce a better image by avoiding shadow or by rotating the thorax to an improved orientation
- Pressing down on the abdomen. This may be used to displace bowel between the abdominal wall and the uterus or to bring the fetal thorax closer to the transducer. On the other hand, it is desirable to have some amniotic fluid between the thorax and the placenta or uterine wall. Pressure which is enough to cause maternal discomfort is seldom necessary
Segmental sequential analysis
- Horizontal movement in the line of the fetus. This can be used to obtain a series of parallel slices. For example, starting with the 4chV, the transverse views of the great arteries and arches can be obtained
- Rotation of the transducer. Rotation through 90º from the 4chV, for example, will image the long-axis or sagittal views of the heart. Alternatively, slight rotation of the transducer from the apical 4chV can “open out” the left ventricular outflow tract
- Angulation. This can be used to sweep the beam up and down the thorax or to avoid a rib shadow
The transverse sweep
- All the information about the fetal heart necessary for routine scanning can be obtained in a transverse sweep. This is a continuous movement of the ultrasound beam from the fetal abdomen to the inlet of the thorax horizontal movement or angulation of the transducer (or both), depending on the size and depth of the fetus.
- If the fetus is small and deep then use angulation
- If the gestation is late and the fetus is close to the transducer use horizontal movement
- Usually a combination of angulation and horizontal slide is used instinctively
Assesment criteria
The fetal echocardiogram is a detailed evaluation of cardiac structure and function. This assessment involves a sequential segmental analysis of 4 basic areas that include the
- Situs,
- Atria,
- Ventricles
- Great arteries and their connections
Fetal laterality
This analysis includes an initial assessment of the fetal right/left orientation, followed by an assessment of the following segments and their relationships. A split-screen static image can be used for documentation.
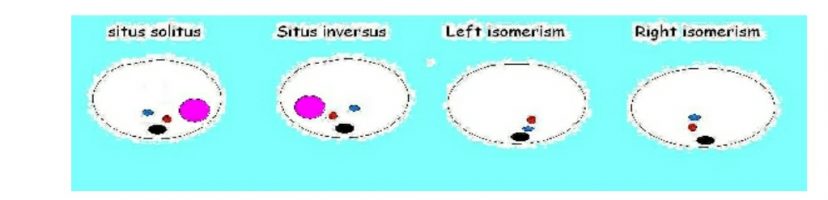
Abdominal Situs
For ascertaining the abdominal situs ,the orientation of fetal head and spine with respect to the right or left of maternal abdomen has to be determined . If the probe is transversely sweeped cephalad from the section of abdominal circumference, stomach and aorta are to the left and cardiac apex is also to the left and slight posterior of the fetal spine. Inferior vena cava is to the right and slightly anterior of the fetal spine.
Solitus: stomach and aorta are to the left of spine and inferior vena cava(IVC) is on right side slightly anterior to aorta.
Inversus: mirror image of solitus i.e. reversed position (stomach and aorta are on right of spine and IVC is on left side slightly anterior to aorta).
Situs ambiguous
Left isomerism (Polysplenia syndrome): IVC is absent at the level of upper abdomen. Most posteriorly located venous structure is the azygous vein which carries blood from lower part of body towards heart
Right isomerism ( Asplenia syndrome): IVC is absent at the level of upper abdomen. Most posteriorly located venous structure is the azygous vein which carries blood from lower part of body towards heart
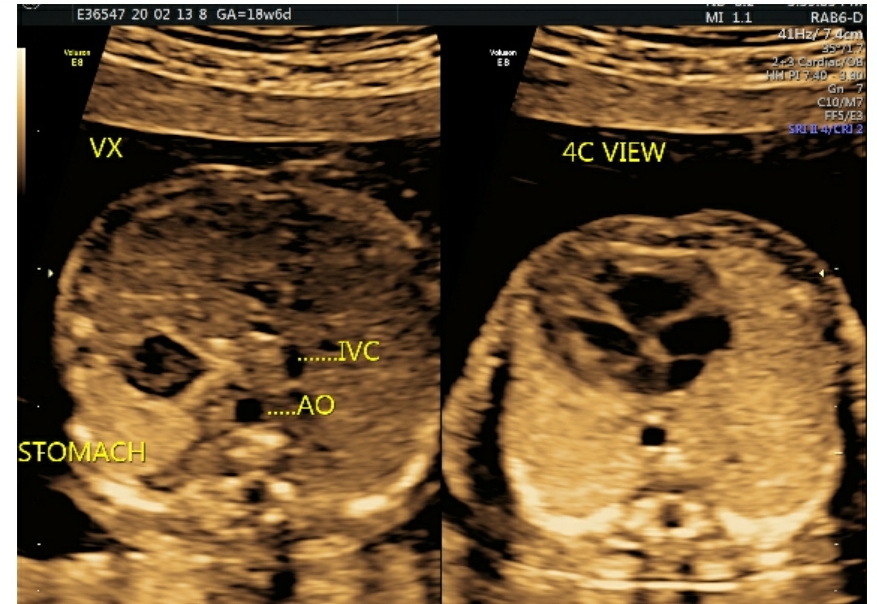
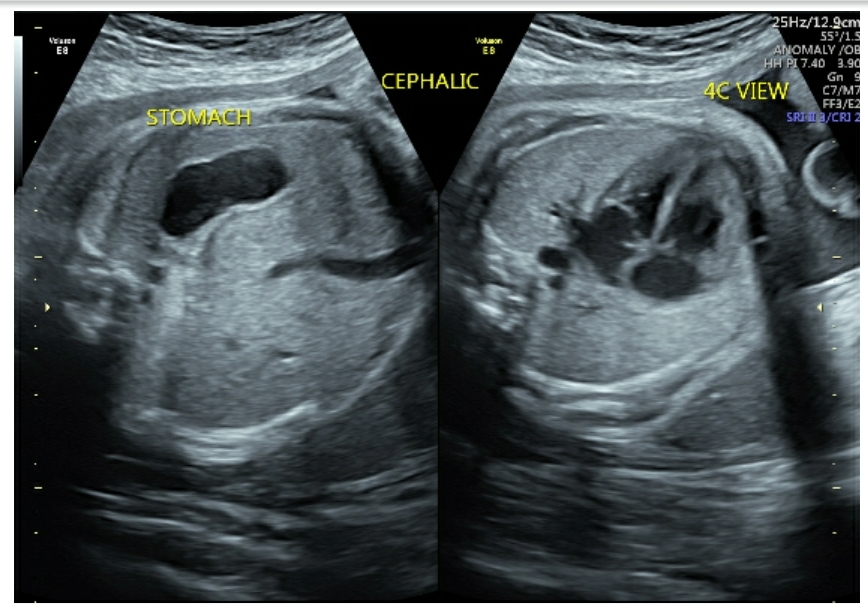
Fig 1a & 1b : Transverse axial view of situs solitus. The split-screen shows fetus in cephalic presentation with the spine posterior. Thus, the fetal left side is closest to the transducer placed on the maternal abdominal wall. These images help confirm situs solitus with the heart and the cardiac apex (arrow) both on the fetal left side.
Cardiac situs:
When we start from abdominal circumference transverse section and sweep cephalad ,the fetal stomach, aorta, cardiac apex and pulmonary artery are towards the same side i.e to the left of the fetal spine.
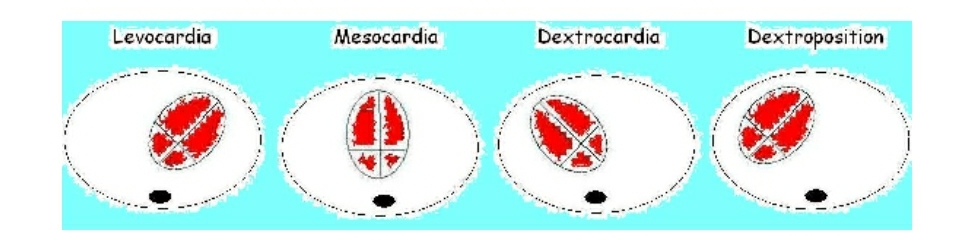
Levocardia-Normal heart is located in left hemi thorax with apex towards left.
Dextrocardia– When heart is located in right hemi thorax with apex towards right. IT IS ASSOCIATED WITH 80-90% CARDIAC ANOMALIES
Mesocardia– When heart is located in middle of thorax with apex towards midline POINTING TO THE STERNUM.
Dextroposition-When heart is shifted towards right side with apex towards left. This is seen in extra cardiac anomalies e.g. – Diaphragmatic hernia.
Cardiac Axis
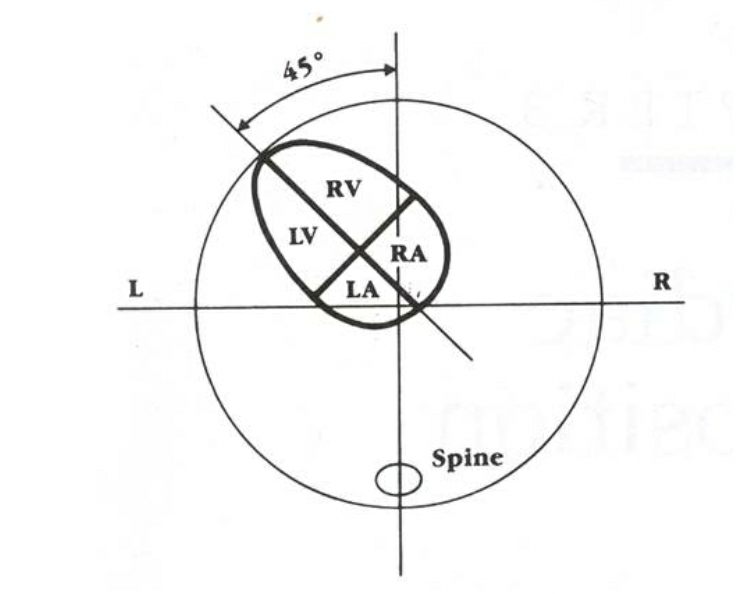
An abnormal cardiac axis in the first trimester at the time of nuchal translucency measurement is an effective tool for detection of CHD.
For e.g. left axis deviation is seen in- Truncus arteriosus, transposition of great arteries(TGA), double outlet right ventricle(DORV), coarctation of aorta(COA),tetralogy of Fallot’s(TOF), Pulmonary stenosis, Ebstein’s anomaly.
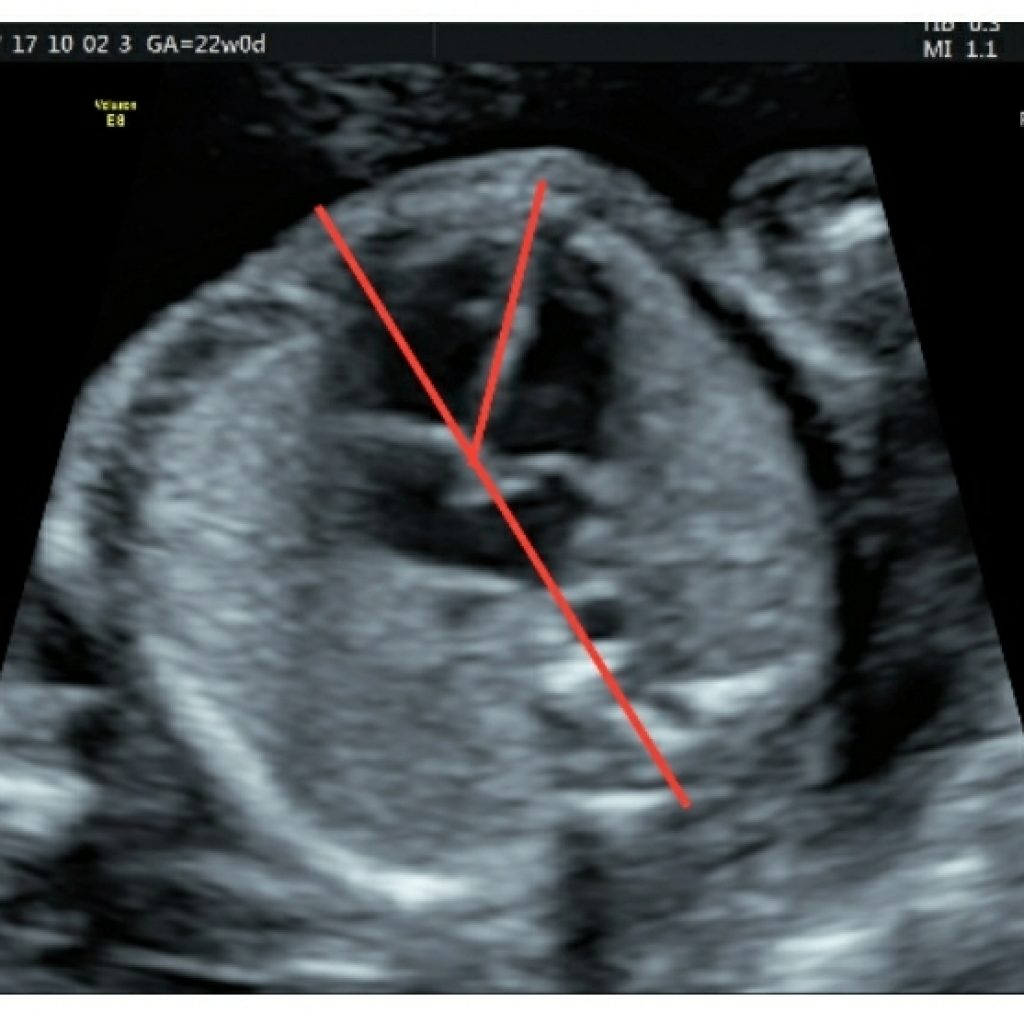
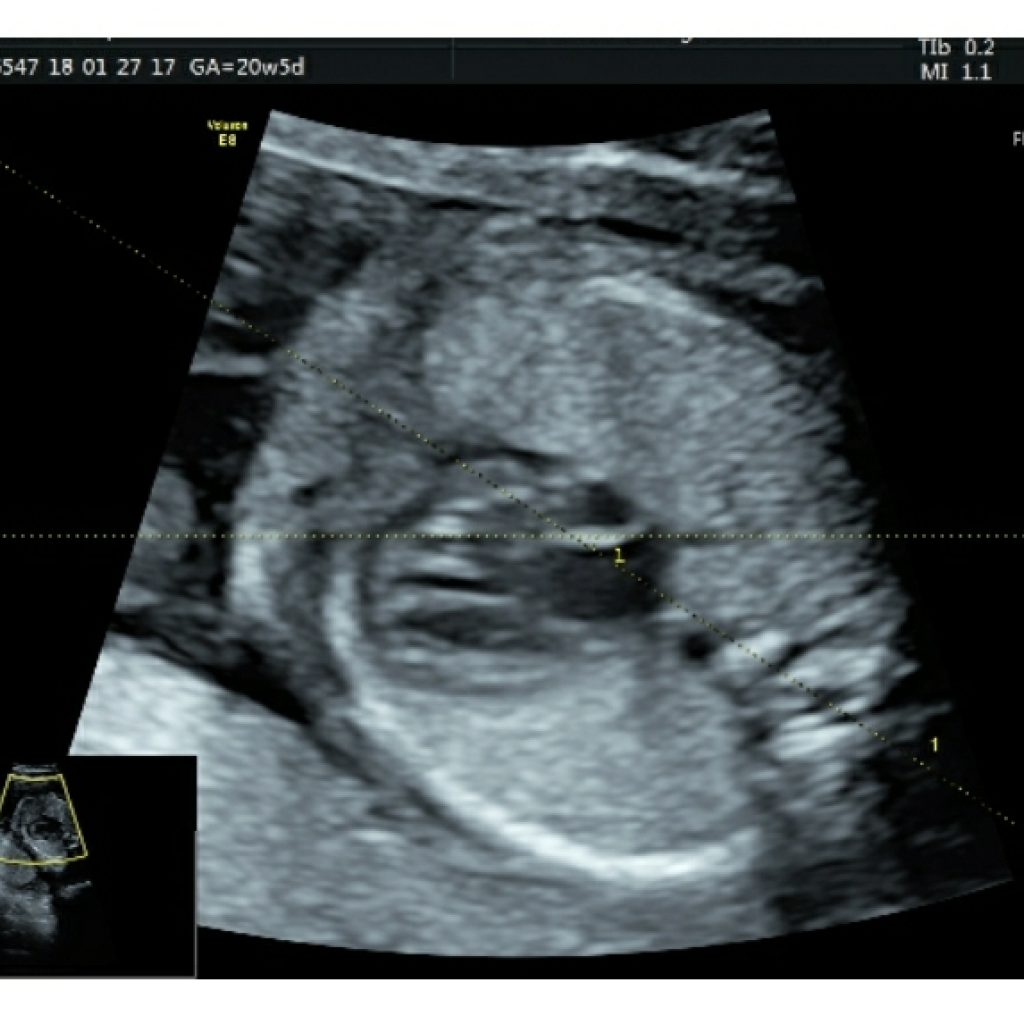
Fig2a,2b: Transverse axial view of the cardiac axis , at the level of the four-chamber view Imaginary line passing through the interventricular septum and another line bisecting the fetal chest from spine to sternum. The cardiac axis is the angle between the two lines. An abnormal cardiac axis is associated with conotruncal heart disease. True axial section is confirmed by the C shape of the rib. If multiple ribs are visible, then scan plane is oblique
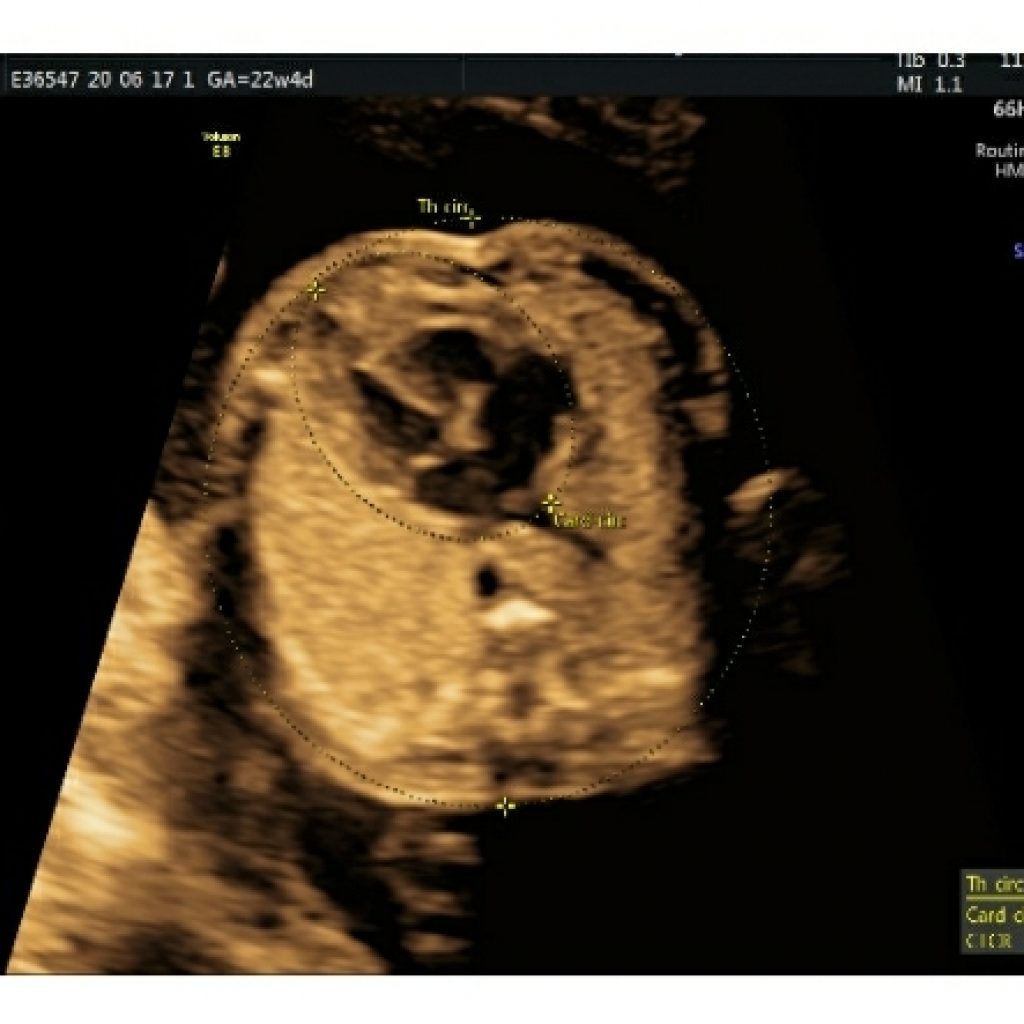
Cardiac size
The heart should occupy approximately one-third of the chest.
Heart area to chest area ratio should be 33%, and heart circumference to chest circumference ratio should be 50%.
These measurements can be easily obtained with the same calipers as those used in measurements of the abdominal circumferences. The thoracic circumference measurement includes the skin.(Fig 3)
Structure and Symmetry :
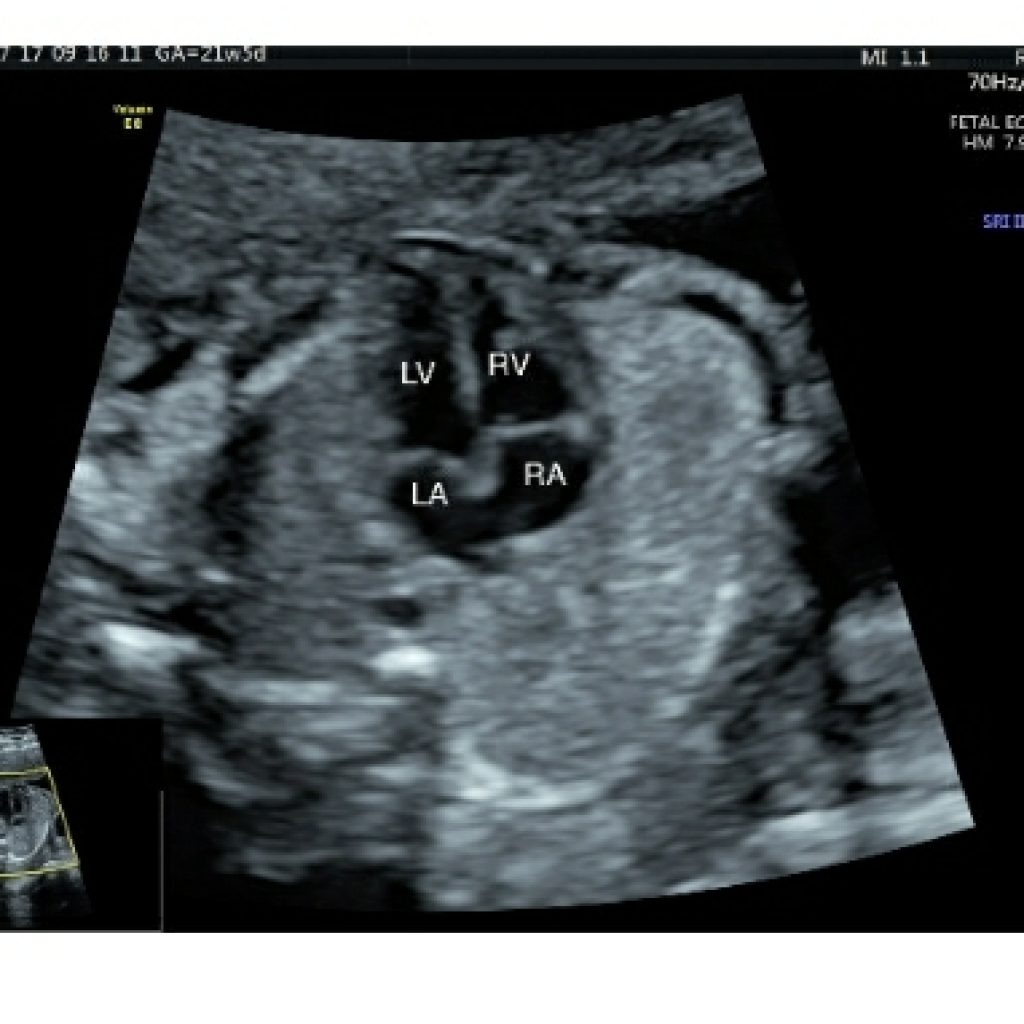
Four chamber view
- When we obtain the abdominal circumference section and sweep cephalad in the thorax ,4 chamber heart view is acquired (fig 4a, 4b).
- The 4-chamber view must be analyzed when imaging the crux (or centre) of the heart.
- If imaging just below the level of the crux, the coronary sinus is seen, if imaging just above the crux, the left ventricular outflow tract comes into view.
- The 4-chamber view is perfect when the thorax is round and there is almost one complete rib seen. If multiple ribs are seen, the section is oblique.
- It can be assessed in apical, lateral and basal position with respect to the fetal orientation . All these views must be analyzed both in 2D and with color flow mapping (fig 4c).
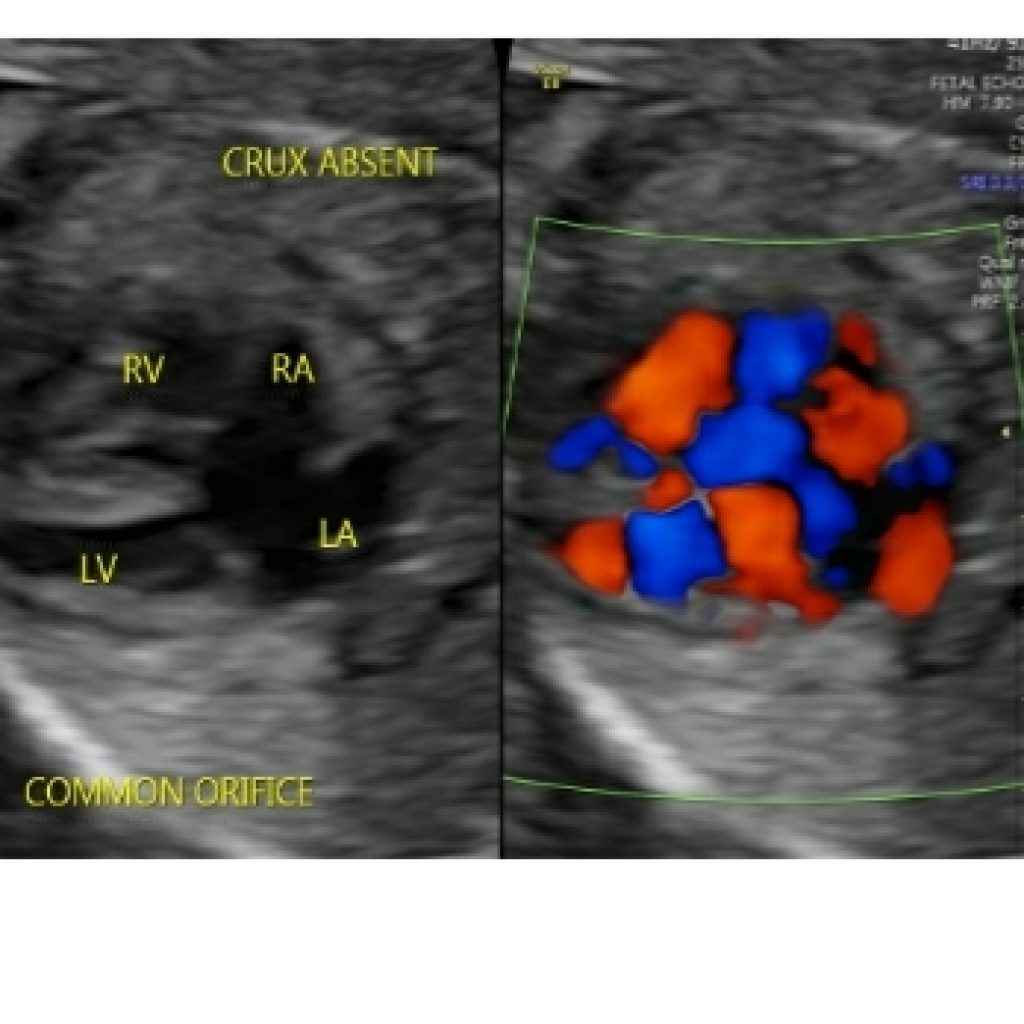
- Lateral view of four chamber is essential to evaluate the integrity of interventricular septum and septum primum of interatrial septum and crux. Crux is the junction of membranous part of the interventricular septum, septum primum of interatrial septum and septal leaflets of the mitral and tricuspid valves.
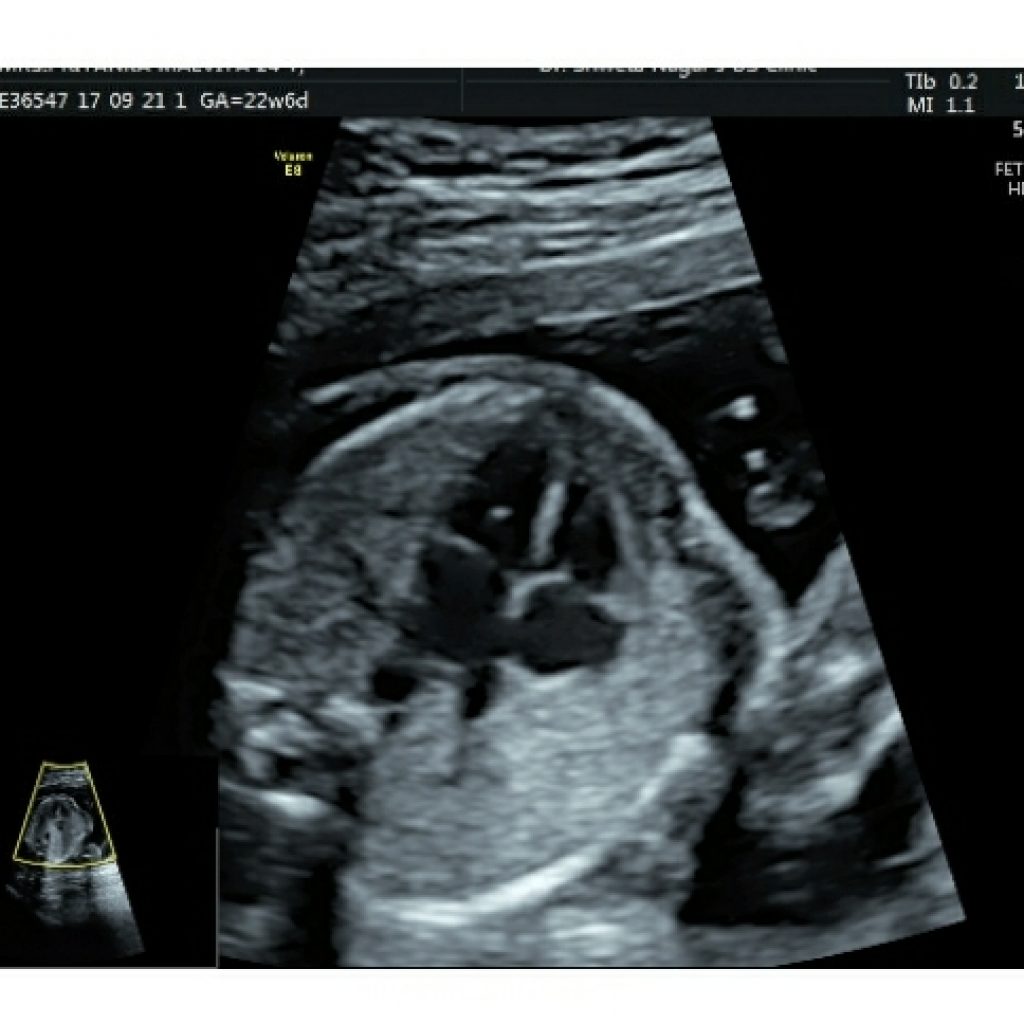
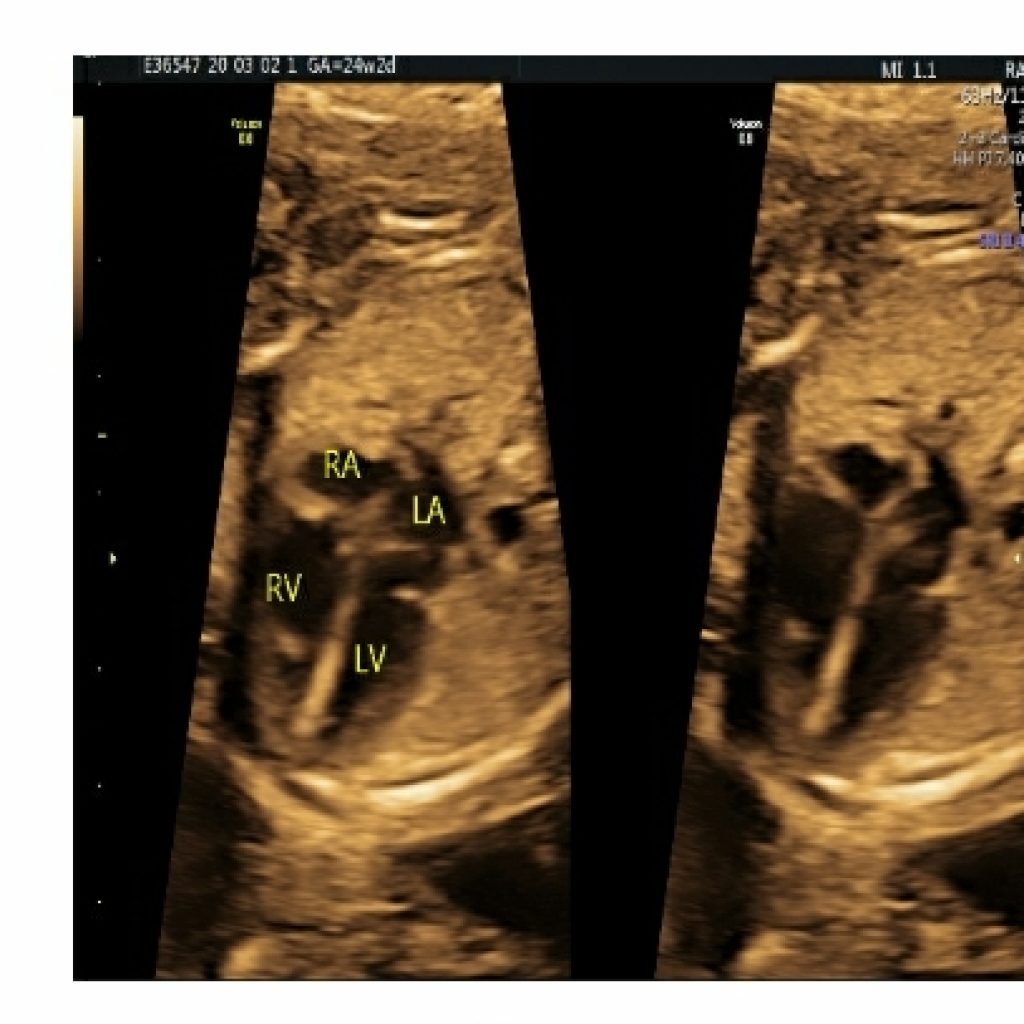
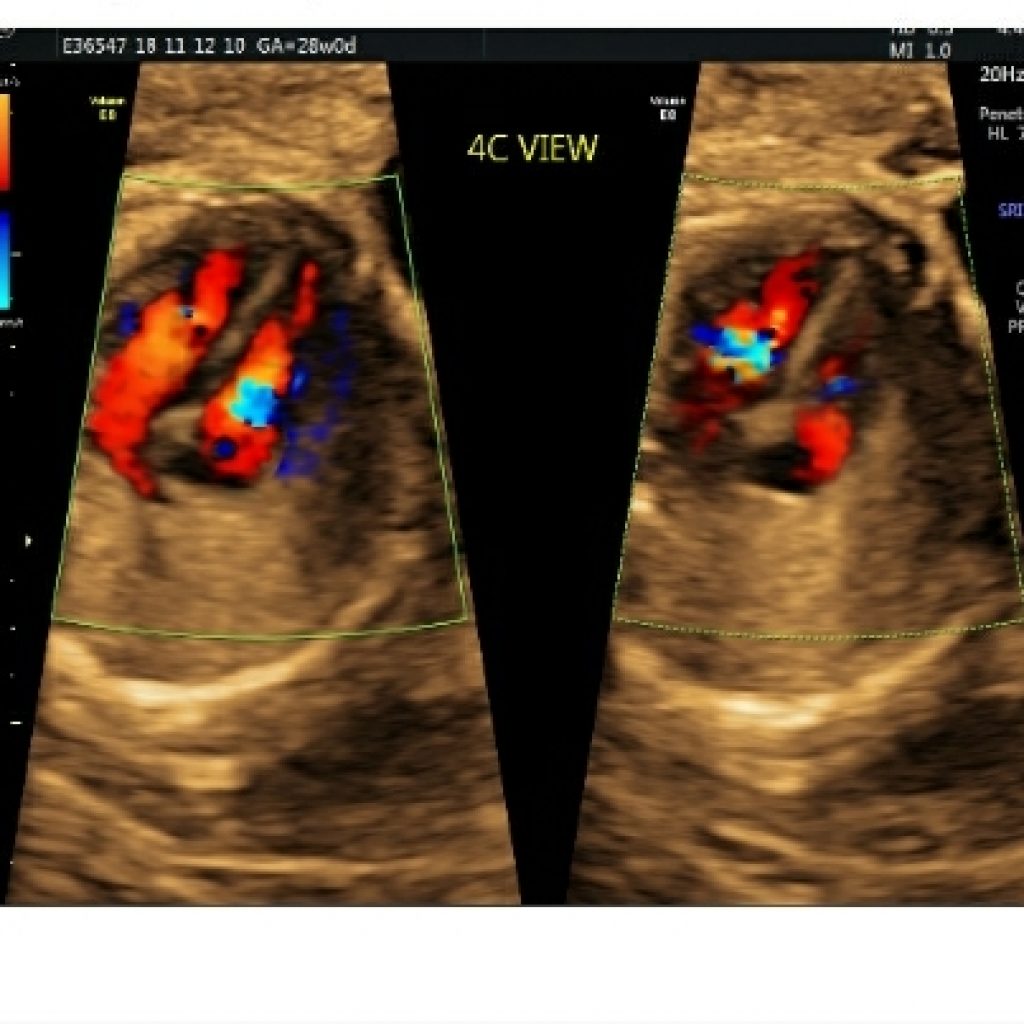
Fig 4a,4b,4c- transverse axial image of fetal thorax with spine in posterior position shows gray scale apical four chamber view, basal view and colour Doppler apical four chamber view demonstrating same and symmetrical column of atrioventricular color flow
The ventricular septum thins from muscular to membranous (fig 5a,5b); this normal change in thickness can be misinterpreted as a VSD. If the US beam is parallel to the septum, an echo drop out artefact may falsely raise suspicion of VSD (fig 5c) ,however if the US beam is focused perpendicular to septum and using color Doppler flow imaging ,it is easier to exclude a VSD at this level with higher confidence .The edges of VSD will be echogenic.
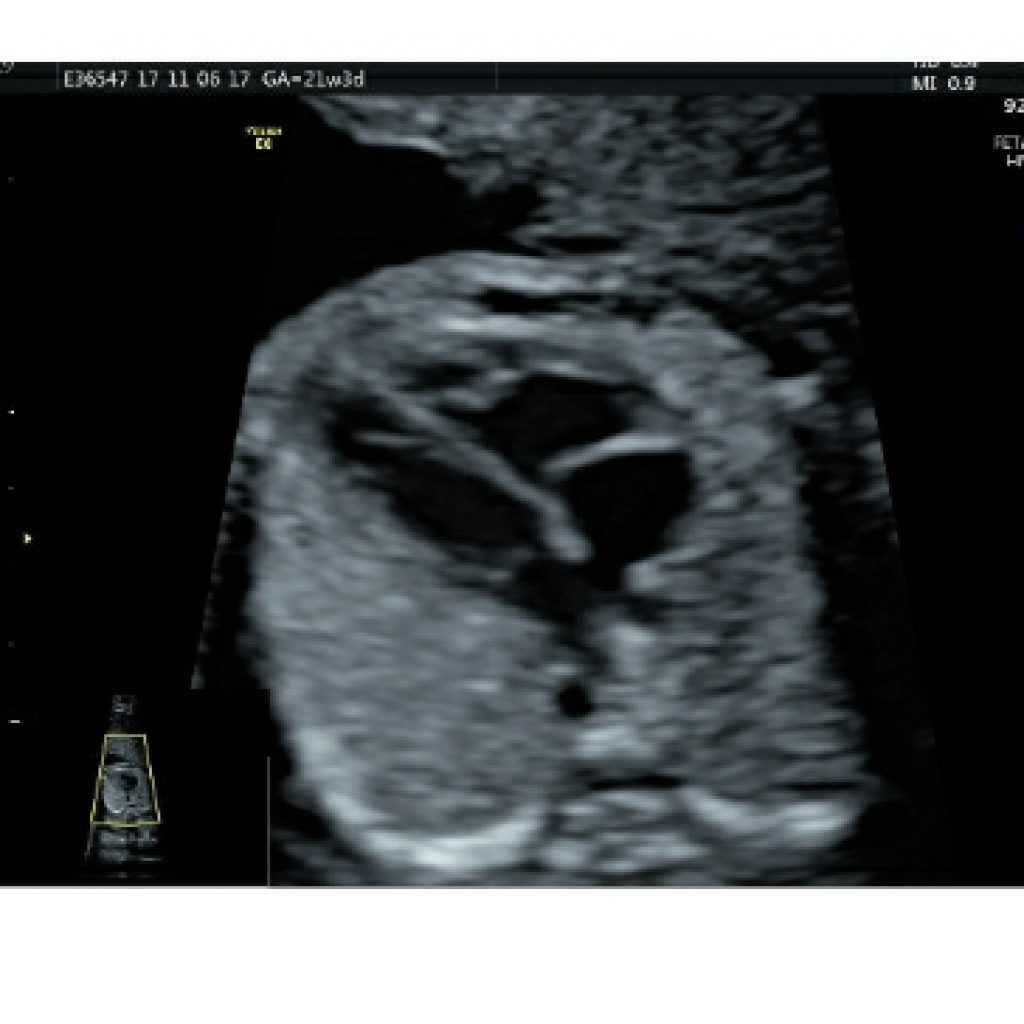
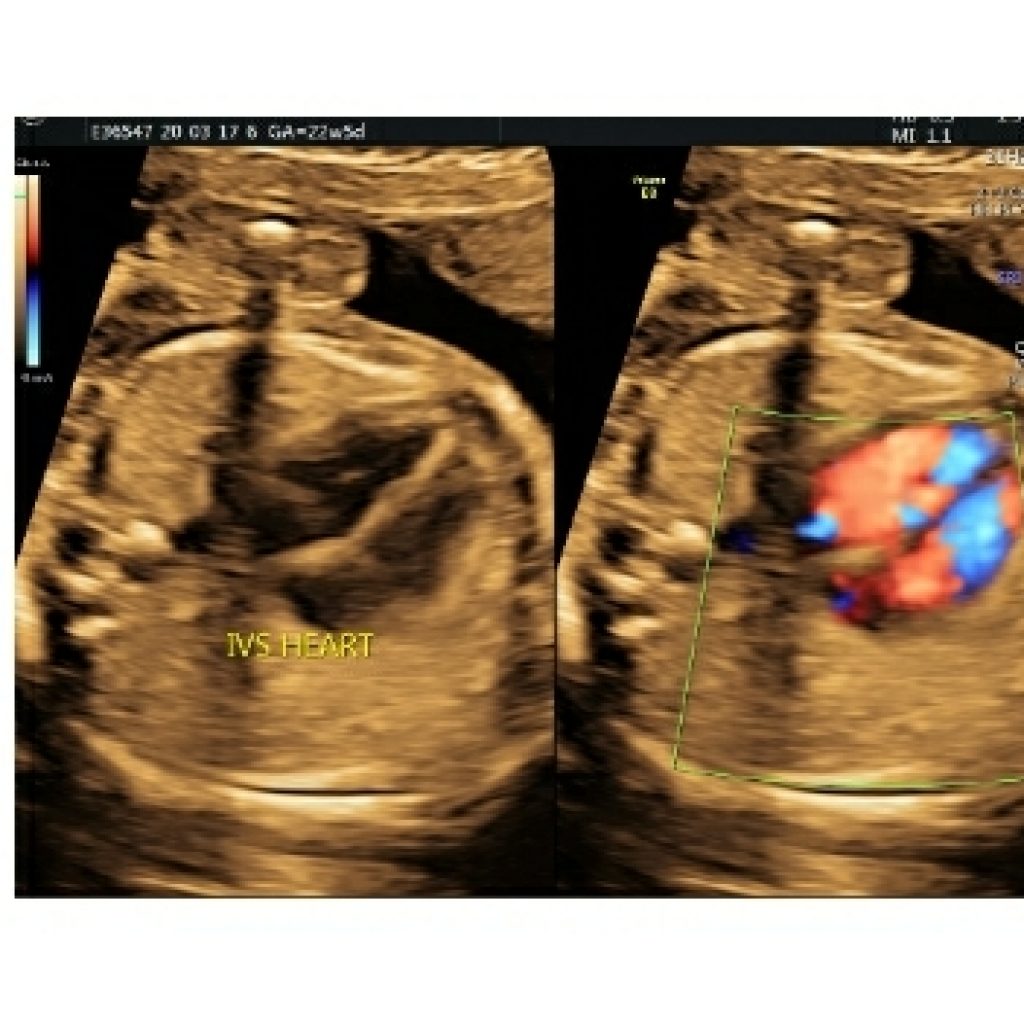
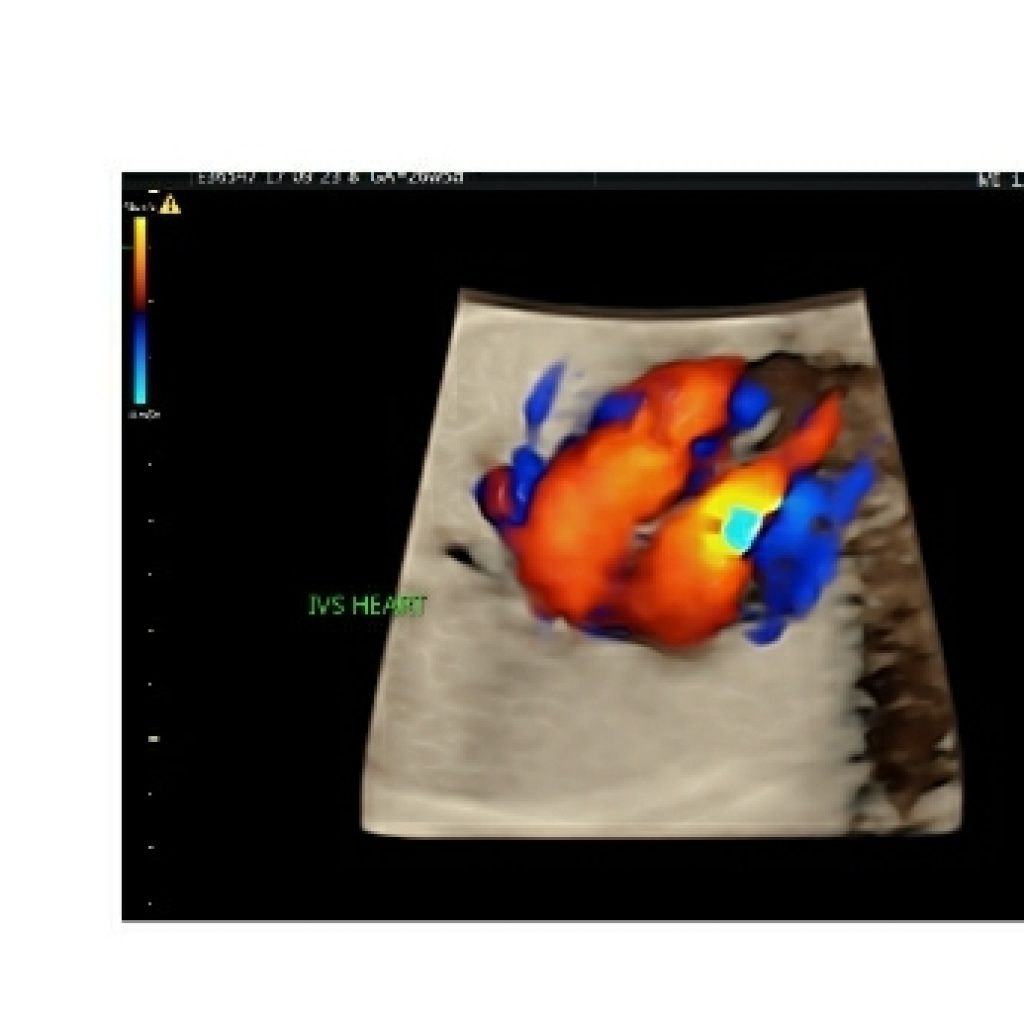
Fig 5a,5b,5c– Transverse axial thoracic view of gray scale demonstrating integrity of interventricular septum,septum primum of interatrial septum and foramen ovale. Colour Doppler and 3DHD live mode demonstrating integrity of interventricular septum.
As the interatrial septum is traced longitudinally cephalad ,the flap valve of foramen ovale is seen (fig 6a). The normal right to left flow across the foramen ovale (fig 6b), which is reversed in lesions of critical left heart hypoplasia.
Pericardial effusion : is important only when fluid is noted along either surface(nondependent portion) of the heart. (fig 6c)
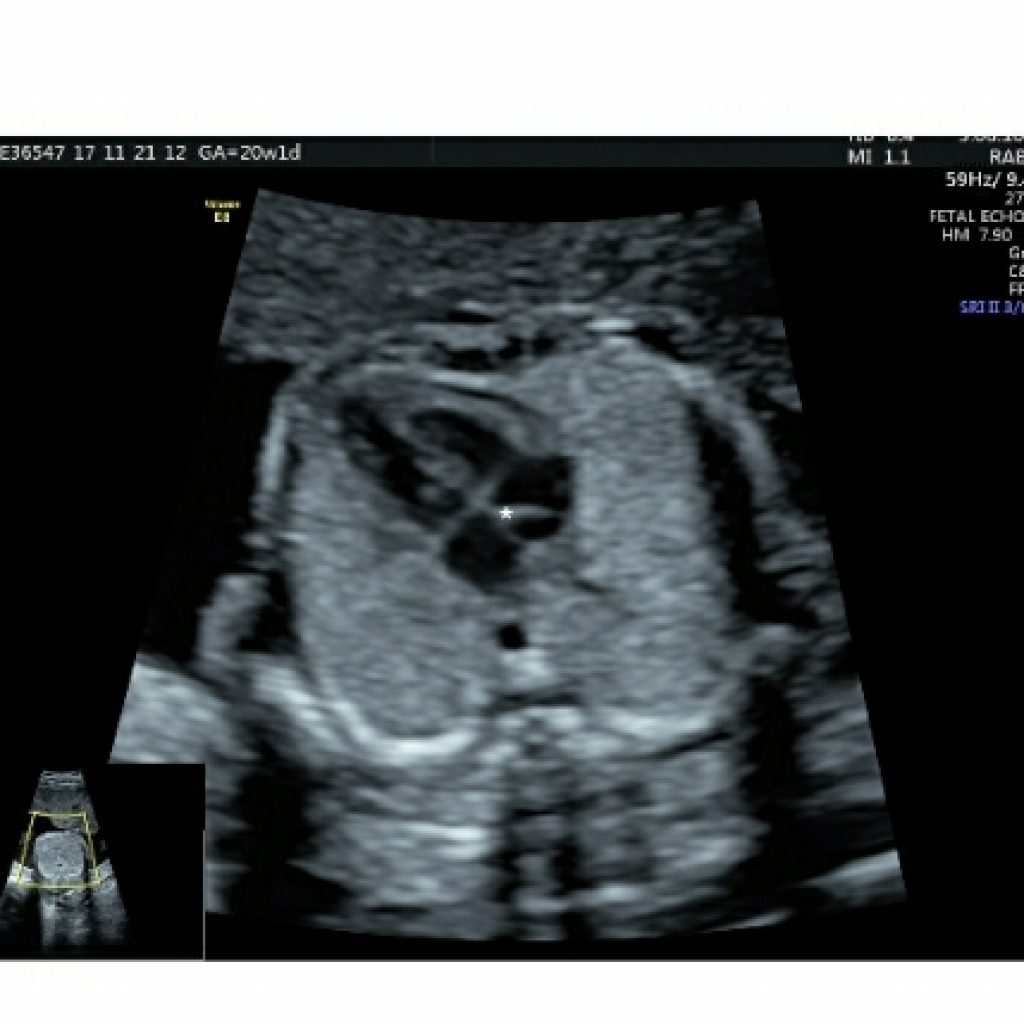
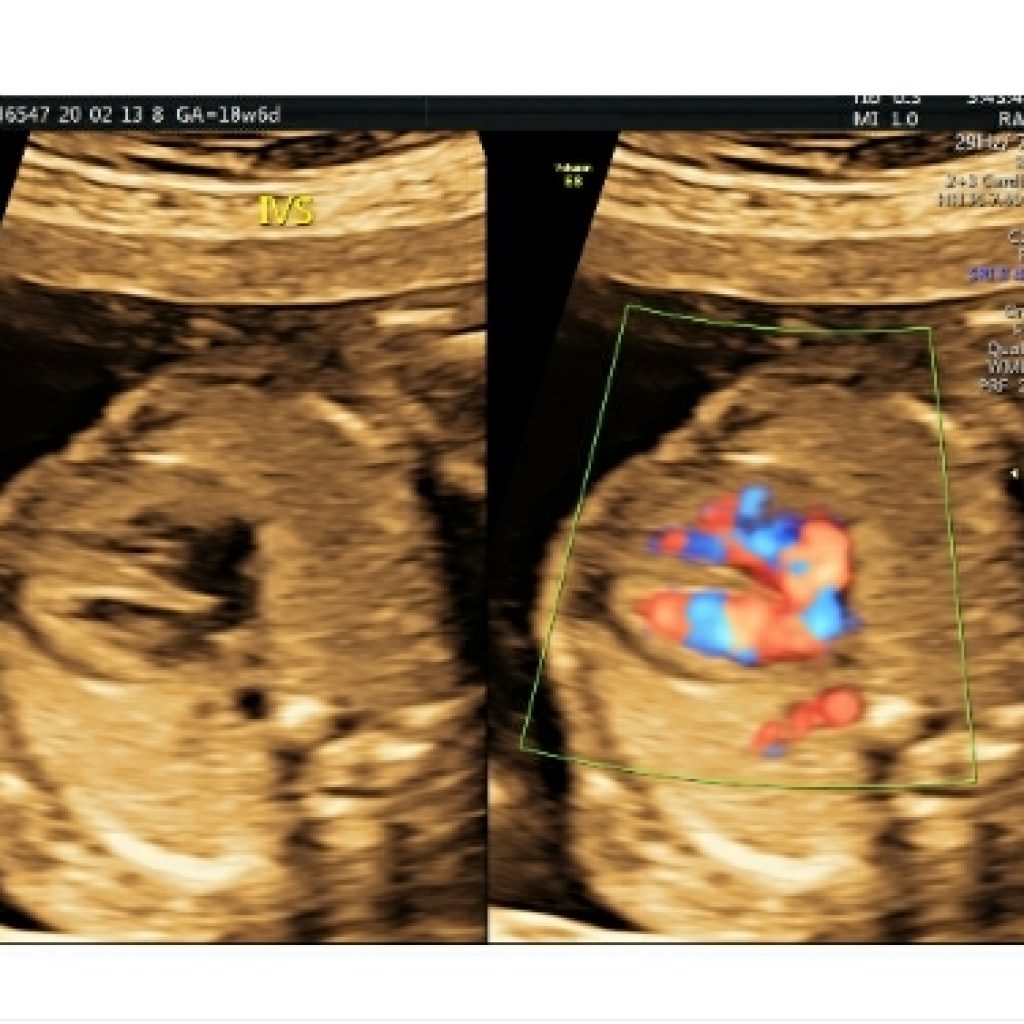
Fig 6a,6b– transverse axial fetal thoracic view asterisk denotes flap valve of foramen ovale (FVFO) and power Doppler mode shows normal right to left shunt across foramen ovale.
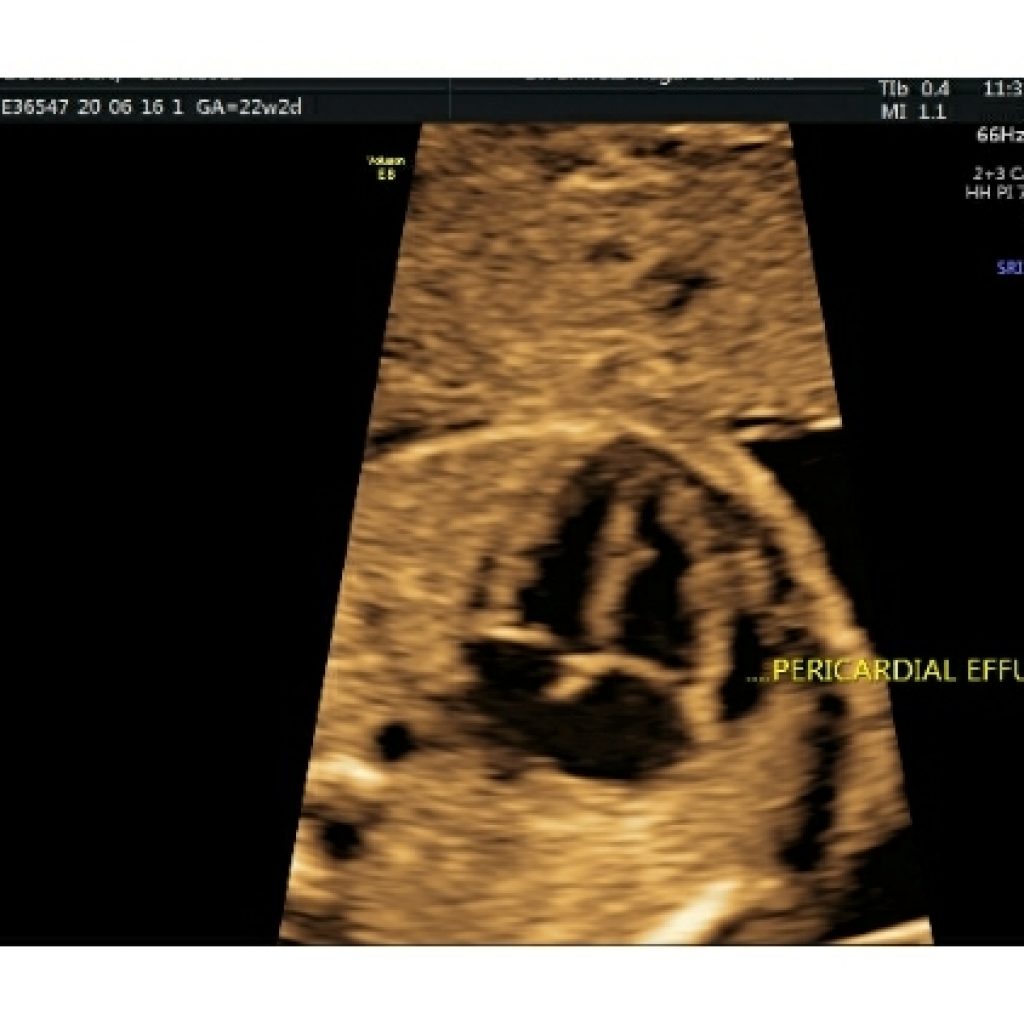
Fig 6c- transverse axial fetal thoracic image shows fluid in nondependent part of heart suggestive of pericardial effusion
Both the atria and ventricles are nearly equal in size .The morphological left ventricle has a smooth interior contour and no septal valve attachment. The left ventricle forms the apex of the heart.The right ventricle is closer to the chest wall and has a trabeculated interior.
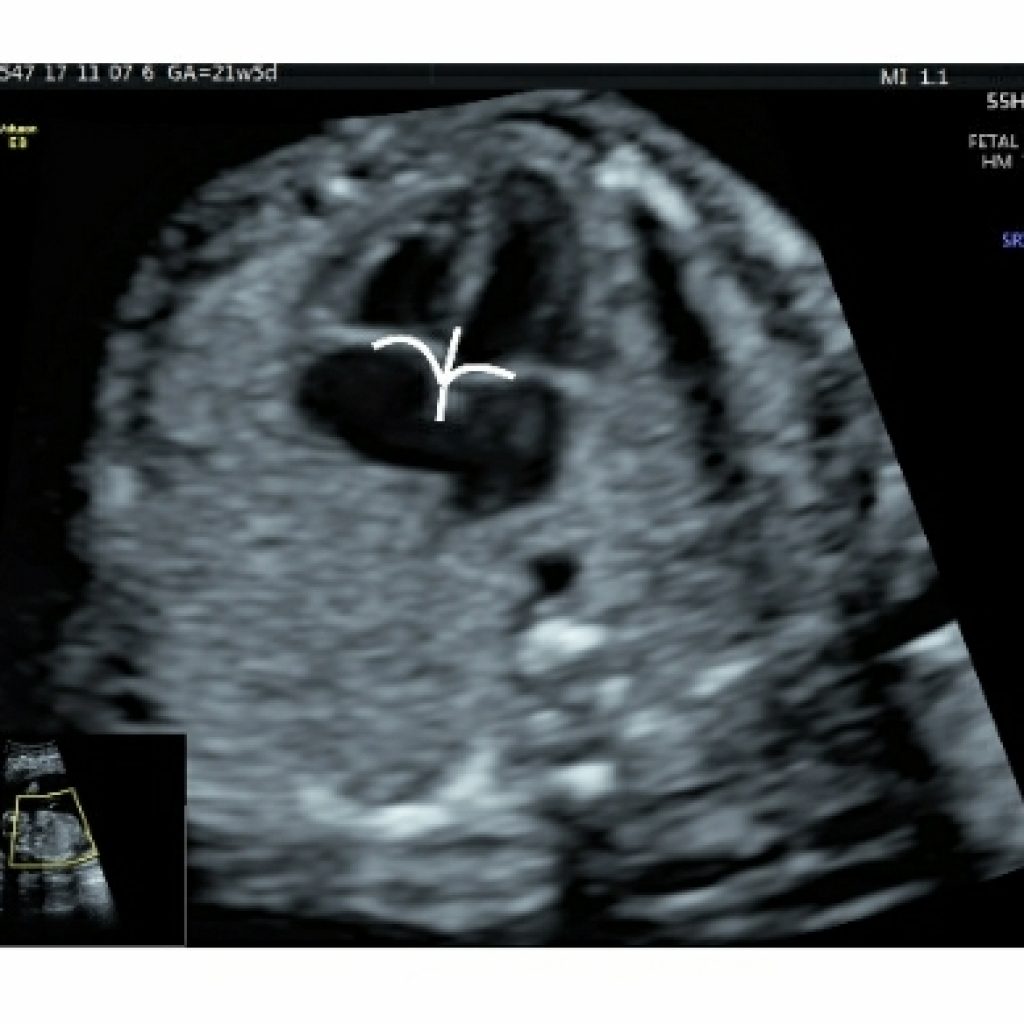
The moderator band (also known as the septomarginal trabecula) is an important landmark for identification of the right ventricle as it traverses the cavity near the ventricular apex to connect the interventricular septum to the anterior papillary muscle.(fig 7a,7b)
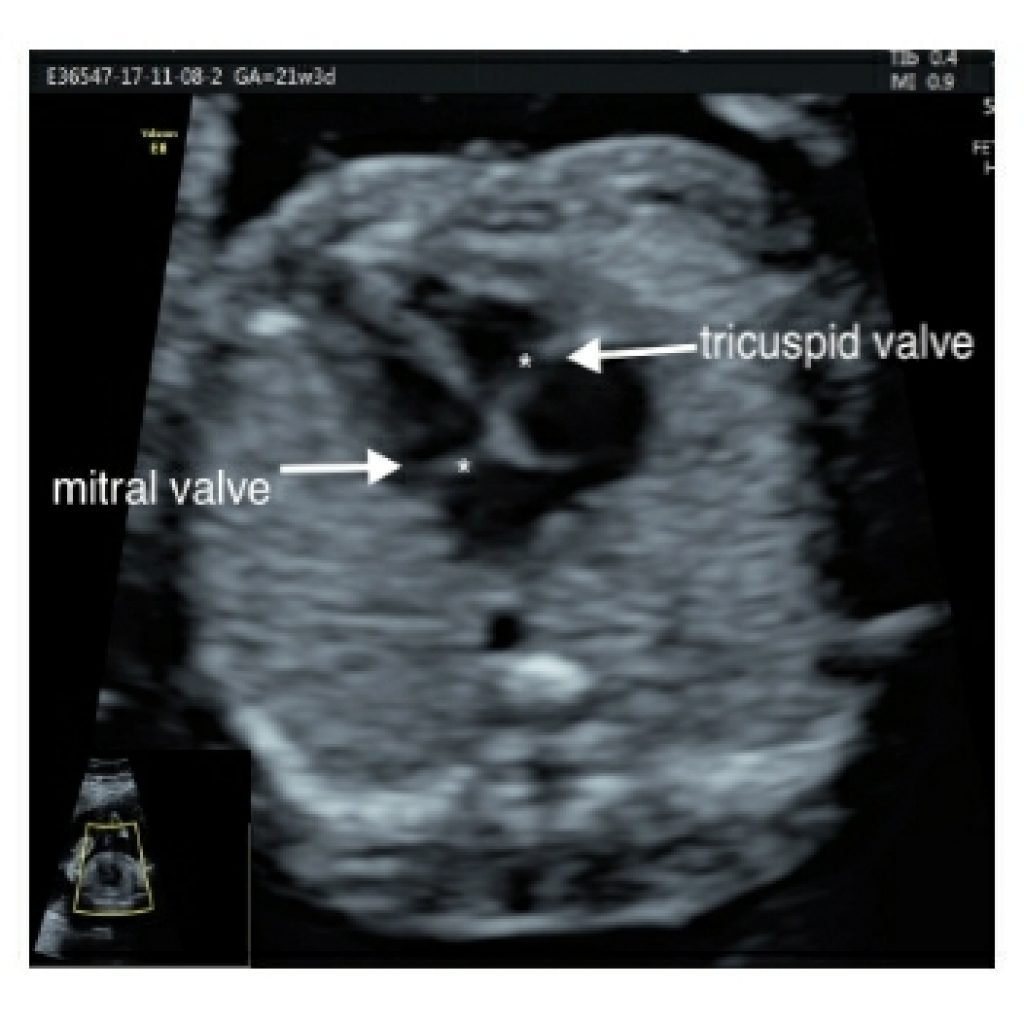
The septal leaflet of the tricuspid valve is more apical in location and attaches to the ventricular septum whereas mitral valve has a free wall attachment. This is called valvular offset (fig 8a).
Abnormal alignment of the atrioventricular valves can be a key sonographic finding for cardiac anomalies such as atrioventricular septal defect (fig 8b).
The valves are always associated with the respective ventricles. There should be equal and symmetrical movement of AV valves and they should be thin in appearance. AV valve function –symmetrical movement of valves /contractility.
LVOT view technique
Maintaining a transverse section of fetal abdominal circumference , sweep the probe cephalad and angulated towards right fetal shoulder.
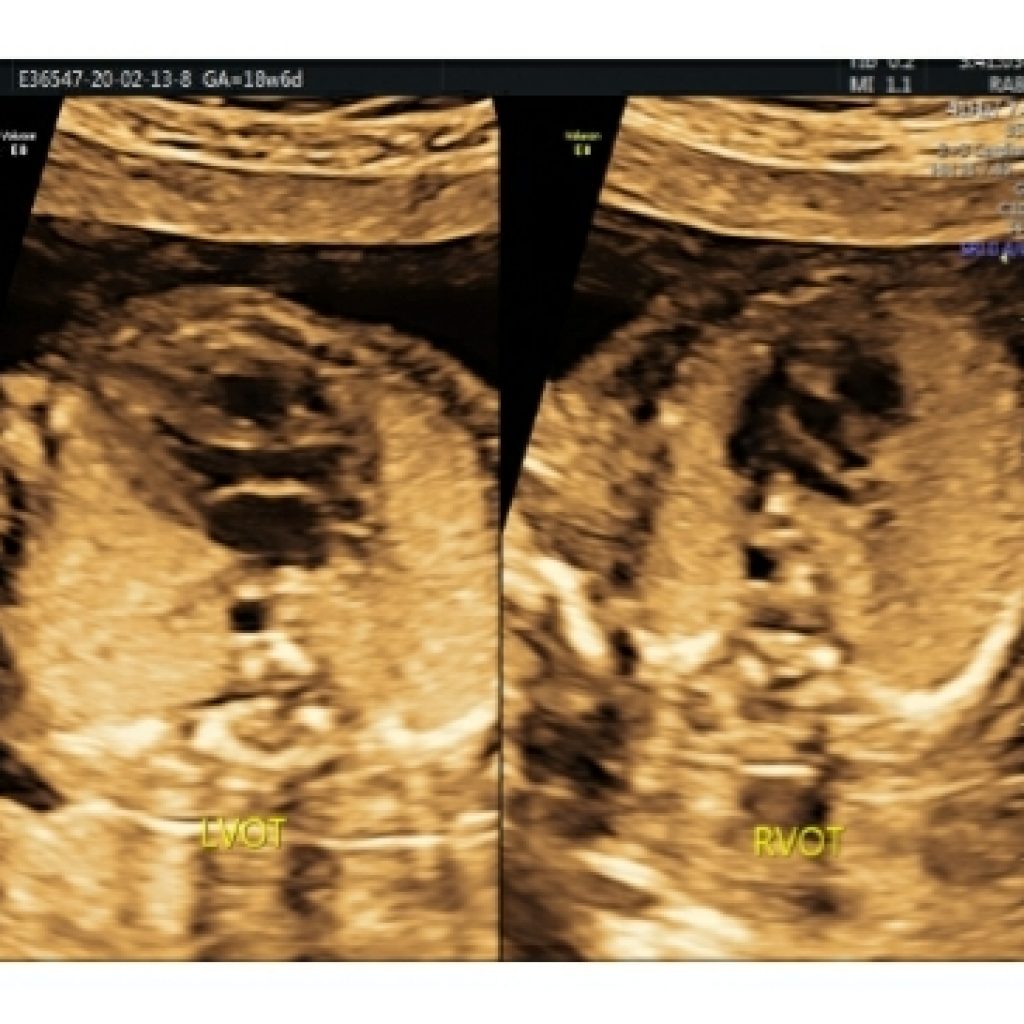
Just above the 4-chamber view, the left ventricular outflow tract is seen which is nonbranching (fig 9a,9b) .
In the normal heart, this displays the aortic origin. and the angle of the aorta to the left ventricle and the continuity of the septum and anterior aortic wall on the LVOT view is called the septoaortic continuity (fig 9c). This has to be checked on colour Doppler as well to rule out VSD (for e.g. in tetralogy of Fallot).
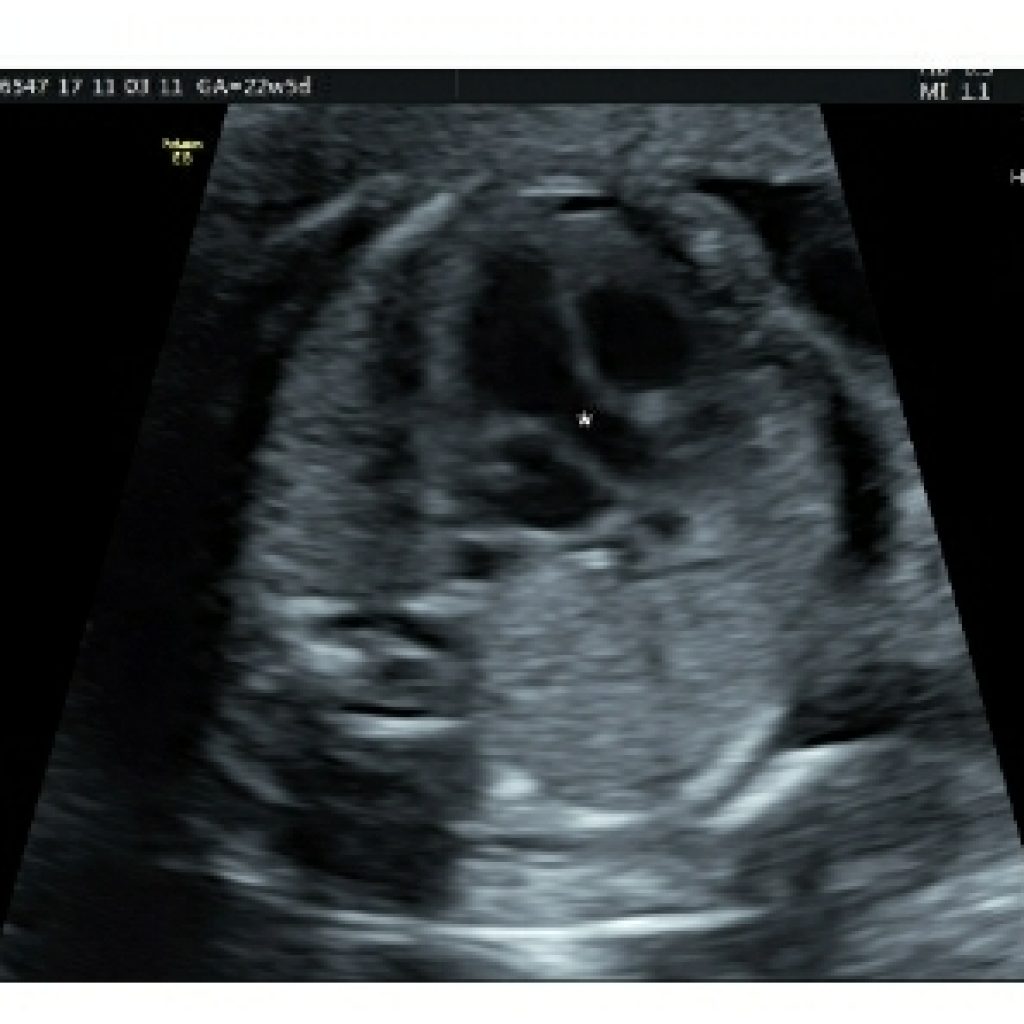
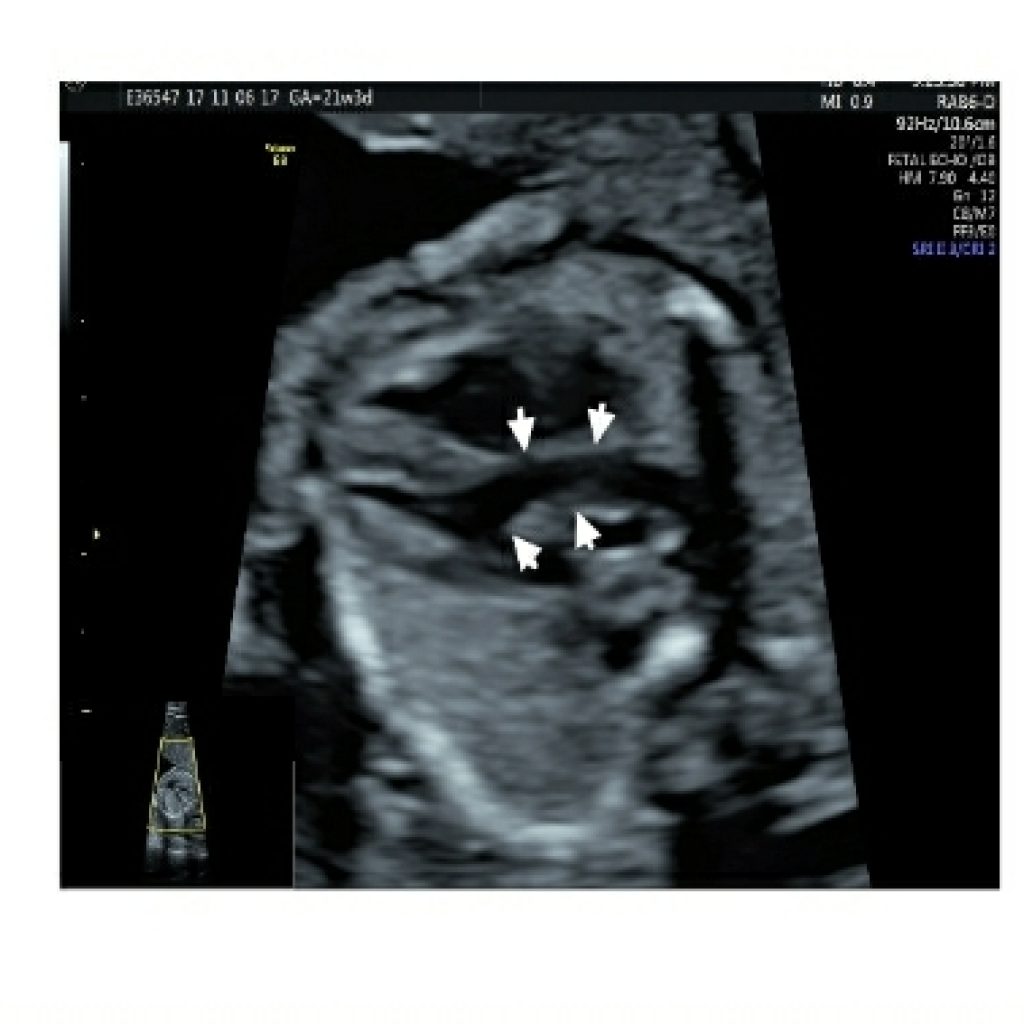
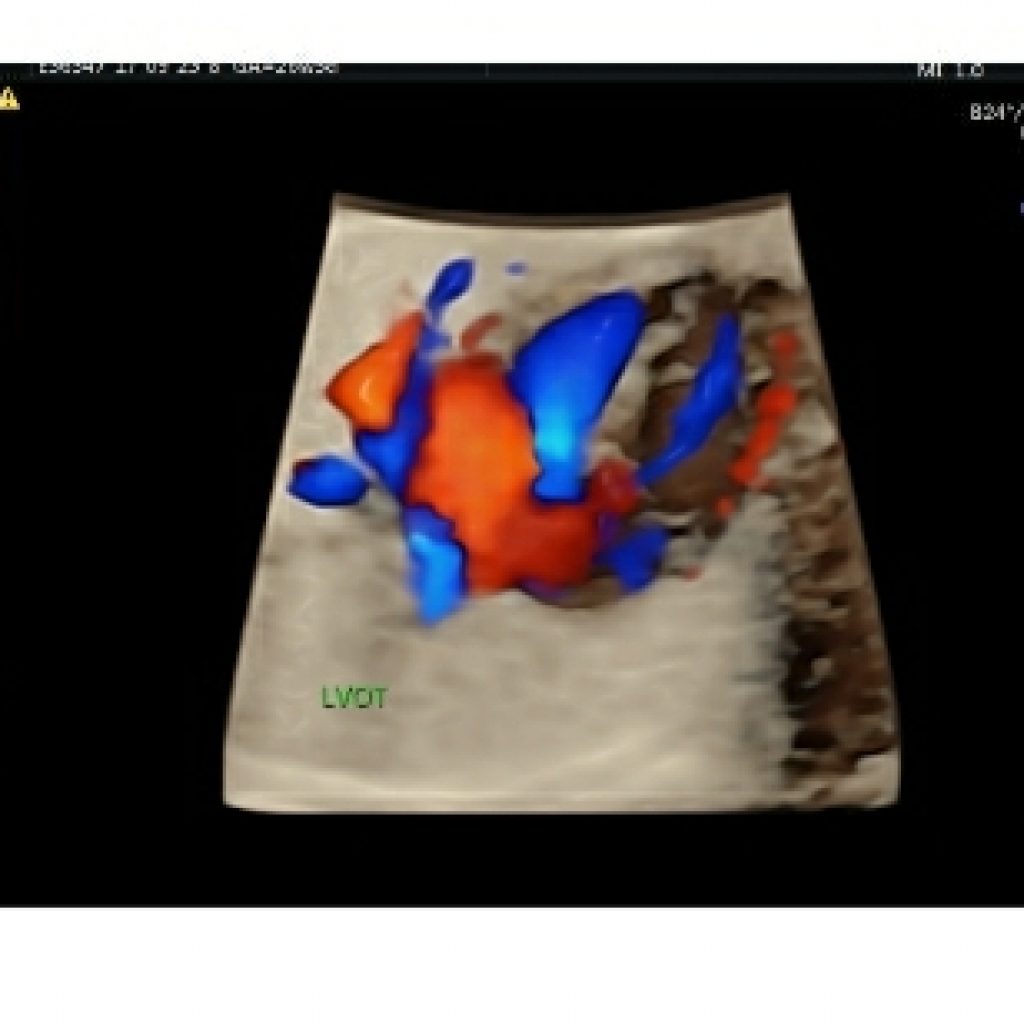
Fig 9a,9b,9c – Transverse axial thoracic view showing gray scale ,colour Doppler and HD live mode in apical and lateral view of fetal heart demonstrates septoaortic continuity formed by atrioventricular leaflet and interventricular septum
RVOT view
Maintaining a transverse section of fetal abdominal circumference , sweep the probe cephalad and angulated towards left fetal shoulder (fig 10).
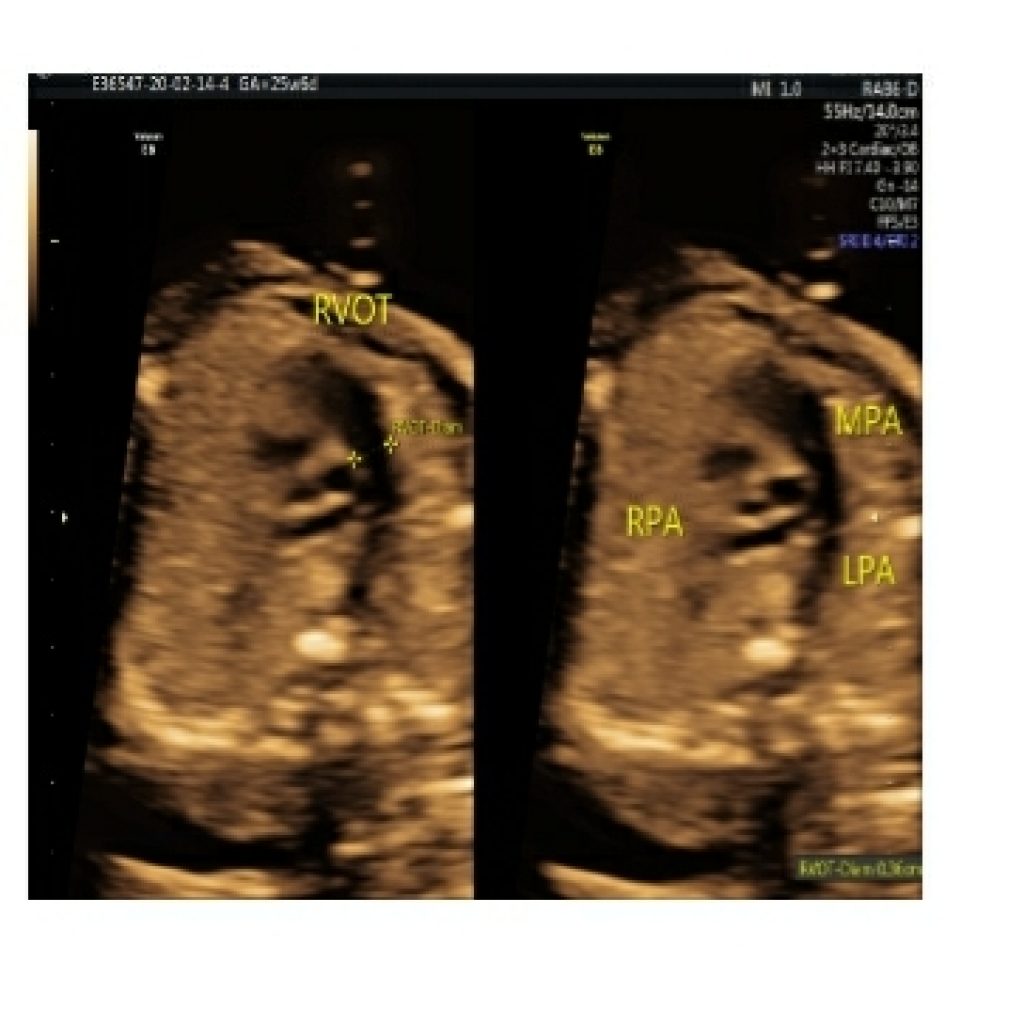
Just above the 4-chamber view, the right ventricular outflow tract is seen which is branching into right and left pulmonary branch arteries (fig 11).
Crossing of the outflow tracts and same colour flow on colour Doppler is important to rule out complex cardiac anomalies (fig 12). If two parallel arteries are seen exiting the heart, the most likely diagnoses are transposition of the great arteries or double-outlet right ventricle.
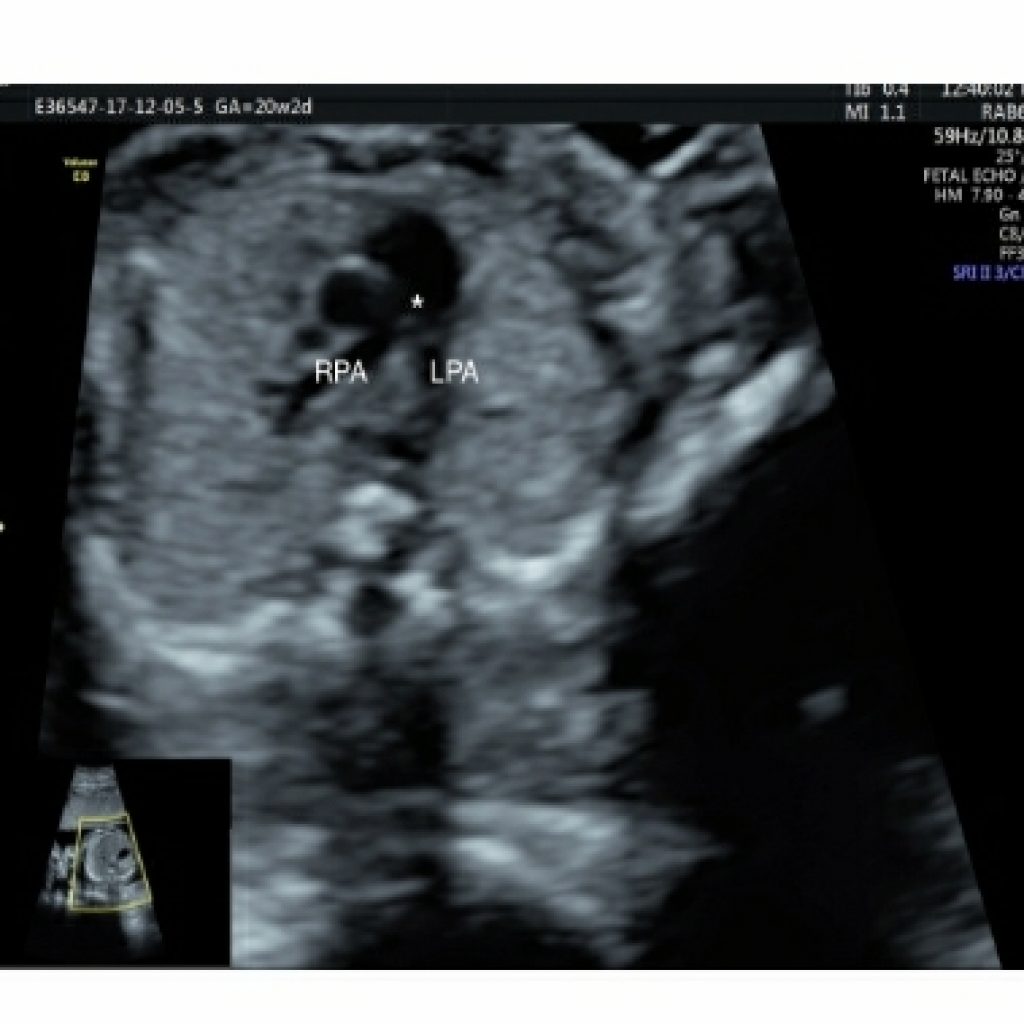
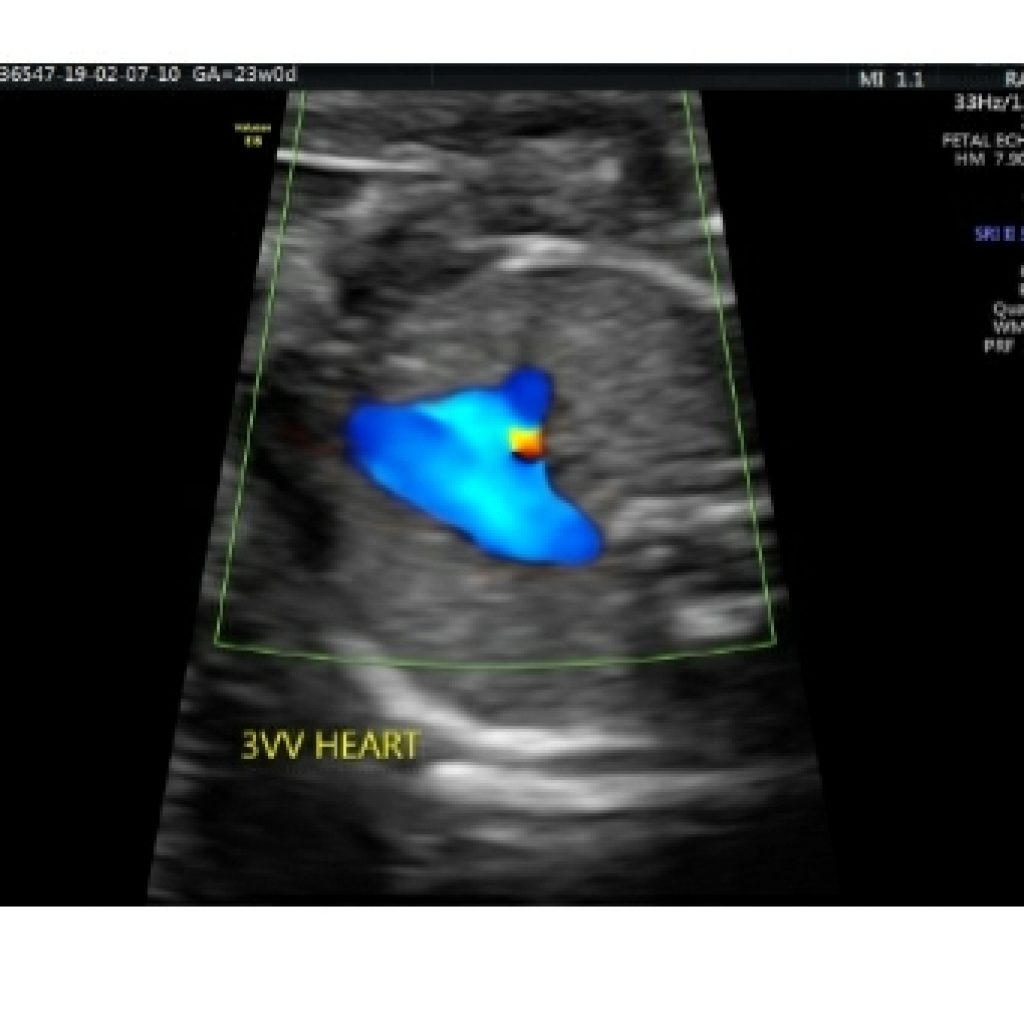
The 3-vessel view
Maintaining a transverse section, sweep the probe cephalad till the fetal neck. Just above the aorta, the pulmonary artery, arising from the right ventricle, crosses over the aortic origin and continues as the arterial duct which can be identified by aliasing of flow when colour box is placed at this level (fig 13).
The right pulmonary artery wraps around the aortic root. From left to right ,pulmonary artery ,aorta and superior vena cava are seen with respective diameters in descending order wherein pulmonary artery is slightly larger than aorta and SVC is the smallest.(fig 14a,14b)
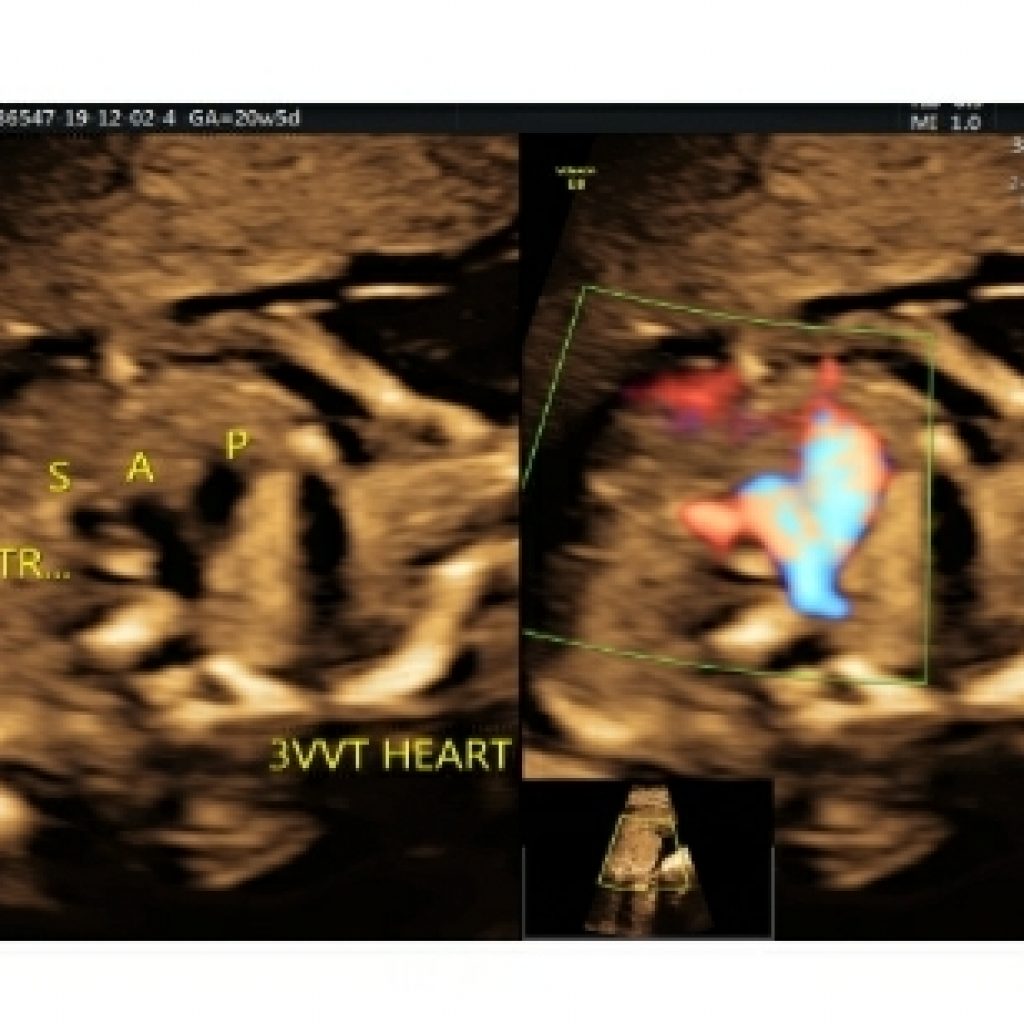
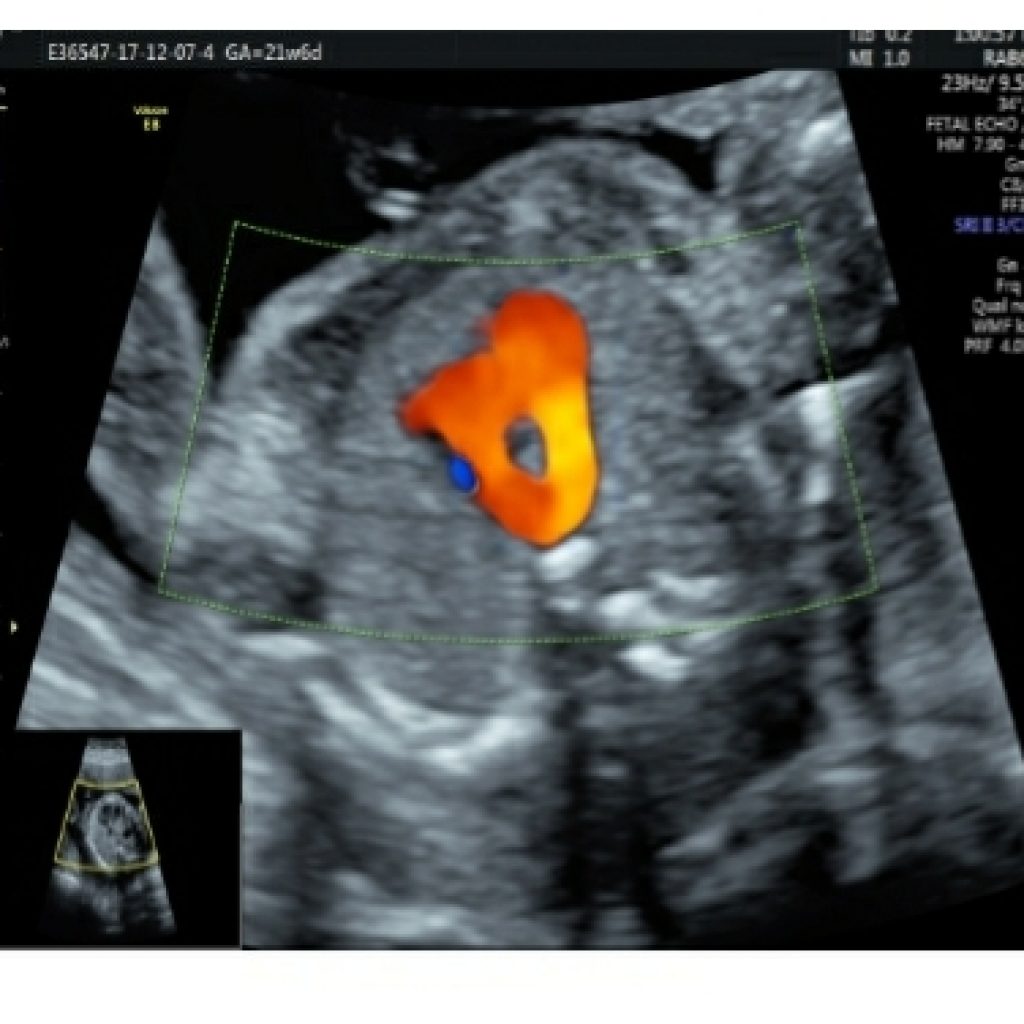
Aorta and pulmonary artery form a V to the left of the trachea.(fig 14a,14 b).
Direction of colour flow should be same in aorta and pulmonary artery. No abnormal vessels should be seen to the left of pulmonary artery.
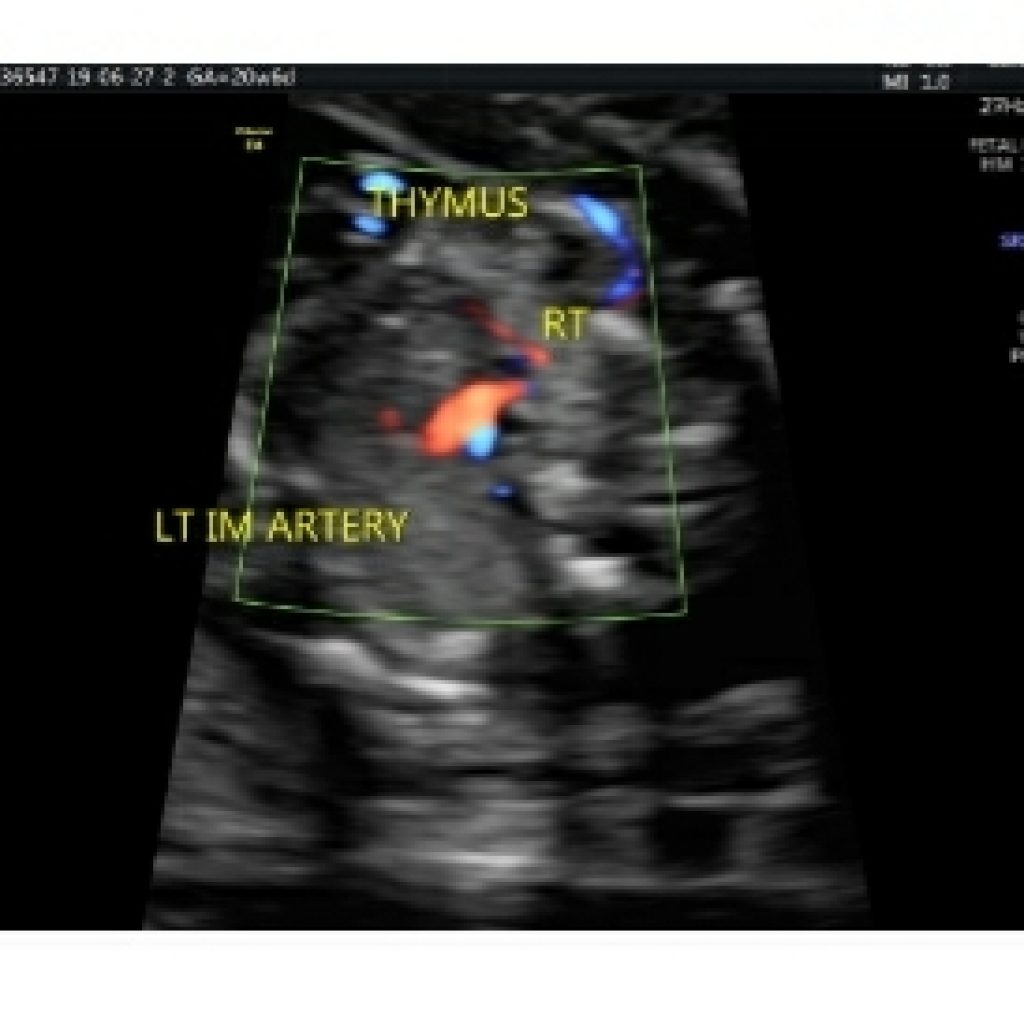
At this 3VT view level, the thymus is bounded by the internal mammary arteries on either side and anteriorly by chest wall. It is important to assess thymic to thoracic ratio (cut off 0.44) to rule out thymic hypoplasia which raises high suspicion of trisomy 21, trisomy 18 and Di George syndrome (22 q microdeletion ) should be ruled out.(fig 15a,15 b)
Area behind the heart
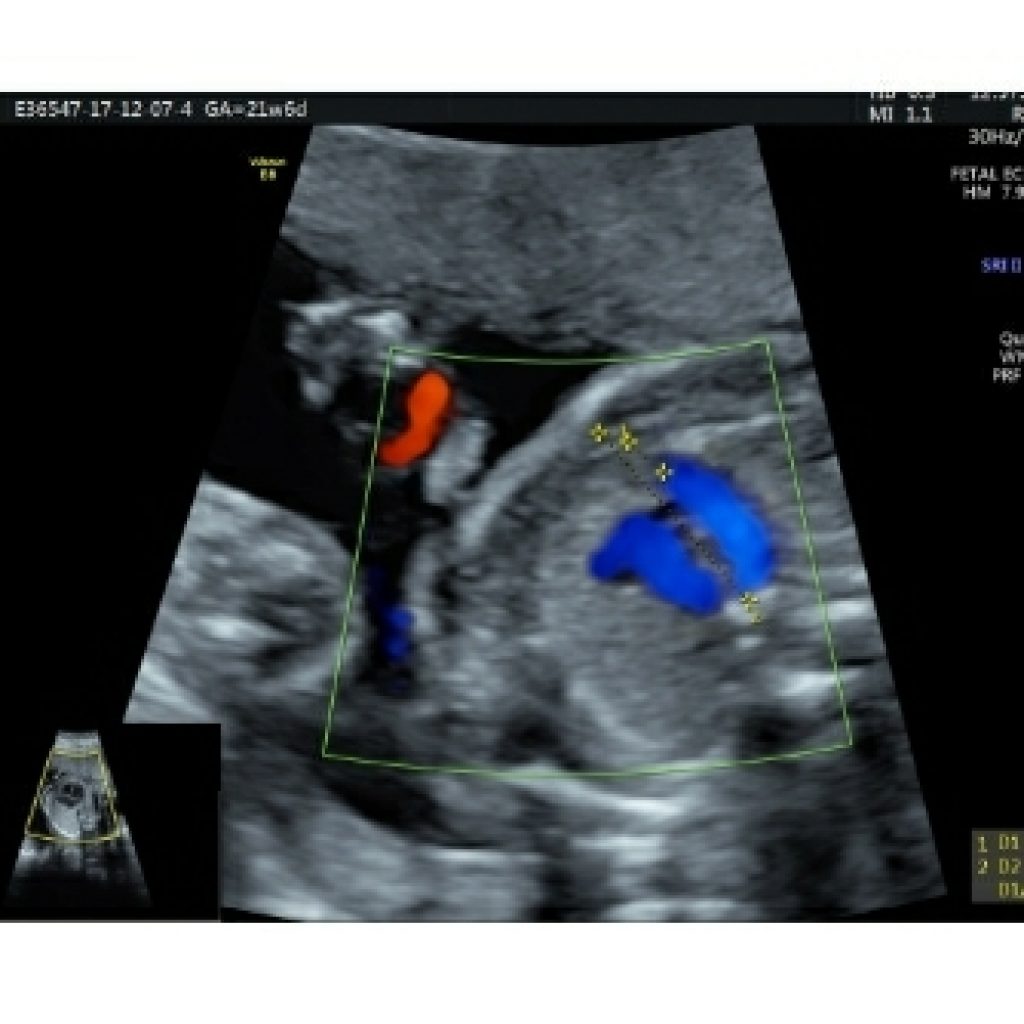
As we sweep cranially from the 3VV, there should be no gap between left atrium and aorta. No abnormal vessel should be seen in this view. As we sweep caudad from this view pulmonary veins are seen forming the left atrium. At least three pulmonary veins should be seen on gray scale or colour Doppler with low flow settings (fig 16a,16b,16c). If there is increased space behind the left atrium or another tubular structure there, think about the esophagus. Fetal swallowing changes the shape and size of the esophagus; therefore, real-time evaluation should allow confident recognition of the esophagus. Persistent vascular structures posterior to the heart may be seen with anomalous pulmonary venous return or with azygos continuation of the IVC. Azygos continuation of the IVC is associated with situs ambiguous and/or heterotaxy syndromes and severe CHD.
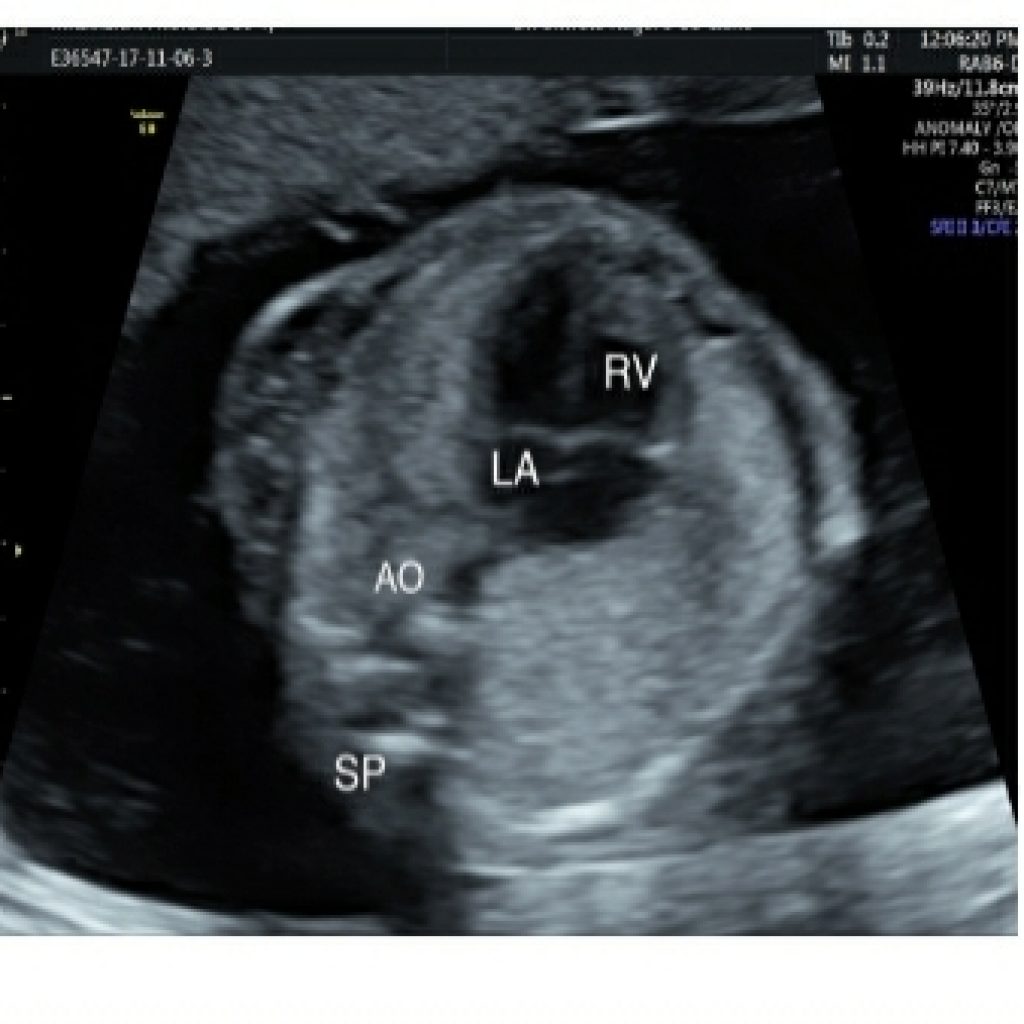
Transverse aortic arch view
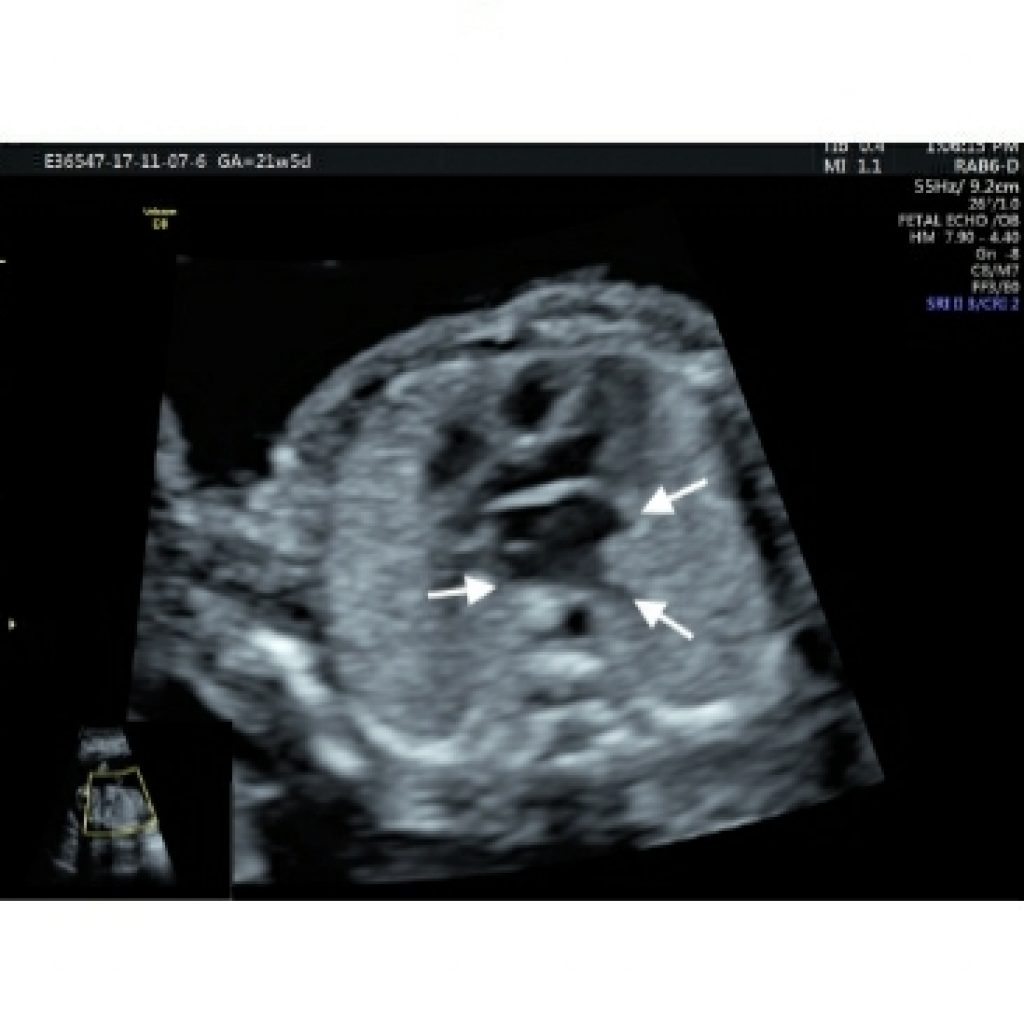
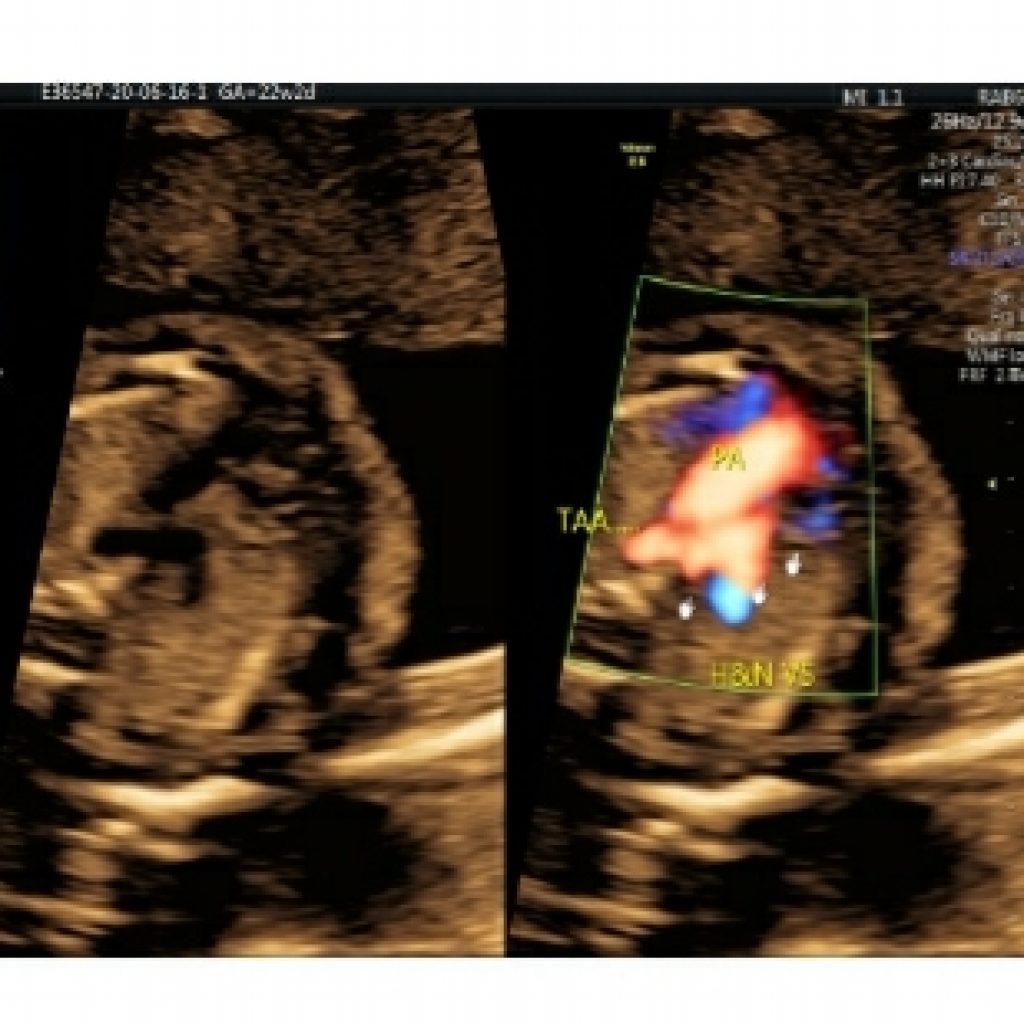
Maintaining a transverse section of the fetal thorax sweep the probe further up towards the fetal neck. Just above the pulmonary artery, the aorta forms the aortic arch with the head and neck vessels in the order of sequence, innominate artery, common carotid artery and subclavian artery (Fig 17).
Followed by this is a narrowed portion of the aorta ,aortic isthmus which is important for ruling out abnormalities, like right sided aortic arch, double aortic arch forming vascular ring around trachea and colour flow reversal (duct dependent flow ) in transverse aortic arch can be indirect sign coarctation of aorta or part of spectrum of hypoplastic left heart syndrome.
The flow reversal in ductus arteriosus guides us to go back to four chamber heart view for right heart abnormalities like pulmonary hypoplasia /atresia.
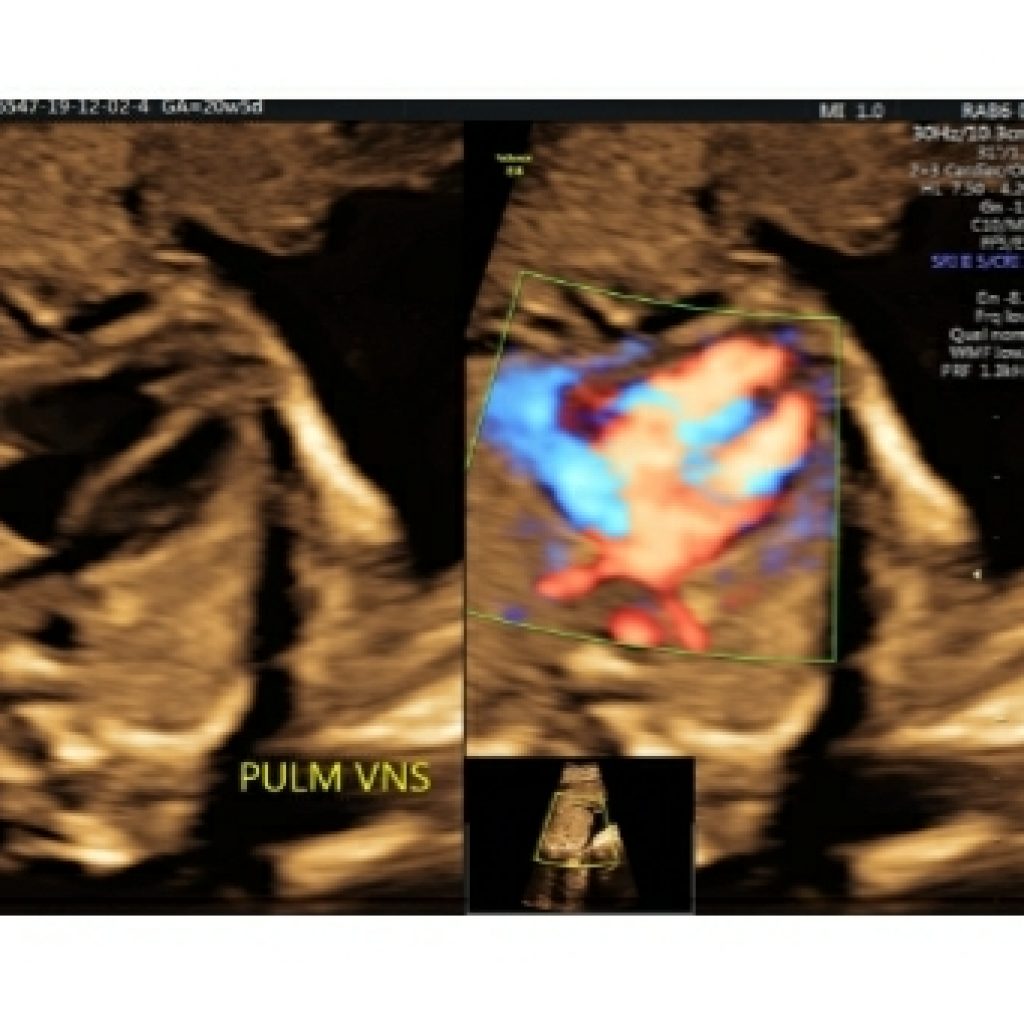
Transverse-oblique views
Slight angulation of the transducer on the transverse aortic arch view shows the arch and duct in the same slice and allows them to be compared for size, position and direction of flow (the V-sign view).
To obtain longitudinal views, the first step is to obtain the 4-chamber view of the heart, the second, to rotate the transducer through 90° and the third, to sweep the beam longitudinally through the fetus, adjusting the transducer position to image the sagittal views of the heart.(fig 18a,18b)
The long axis of the ductal arch: The ductal arch is flat and wide. It can be seen by angulating the transducer anteriorly from the position for the arch of aorta section. The ductal arch starts anteriorly behind the sternum.
The long axis of the aortic arch: The arch of aorta has to be visualized in a sagittal view.It is narrow and round.
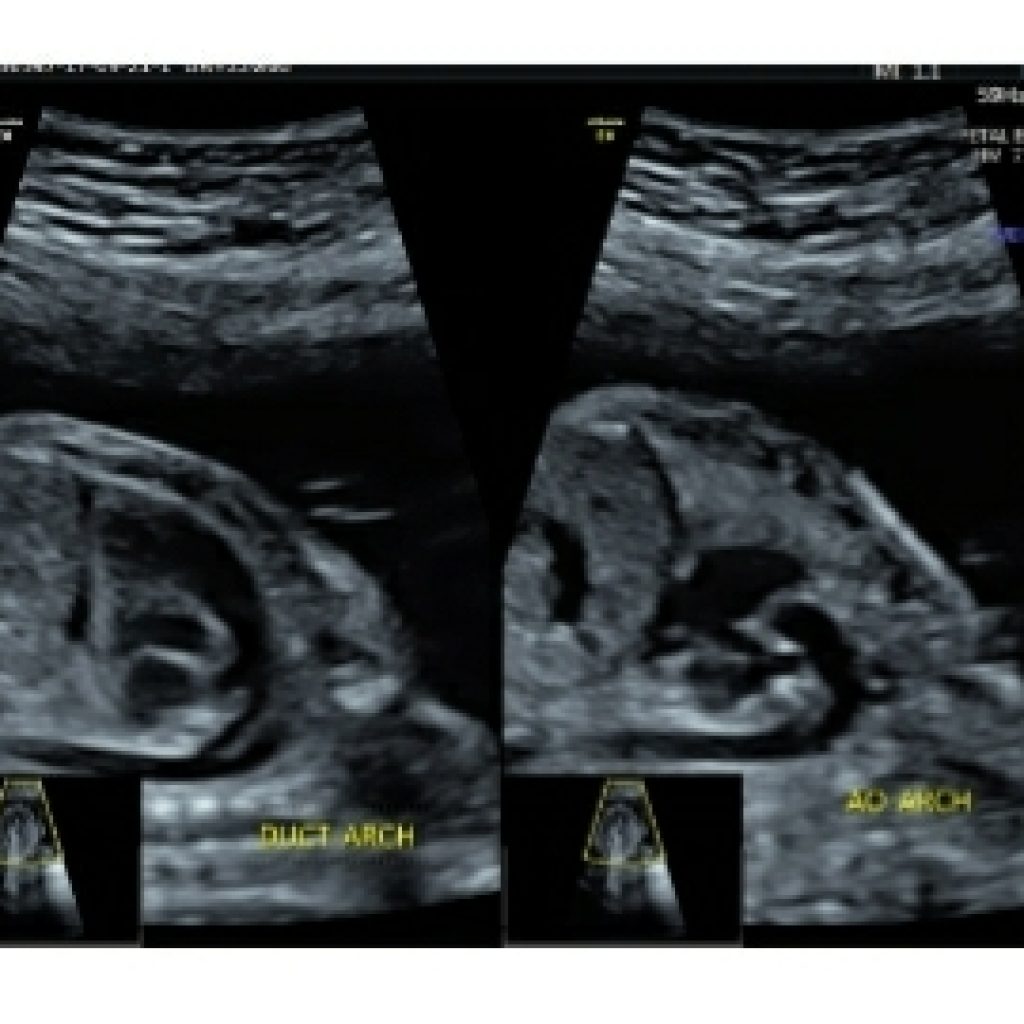
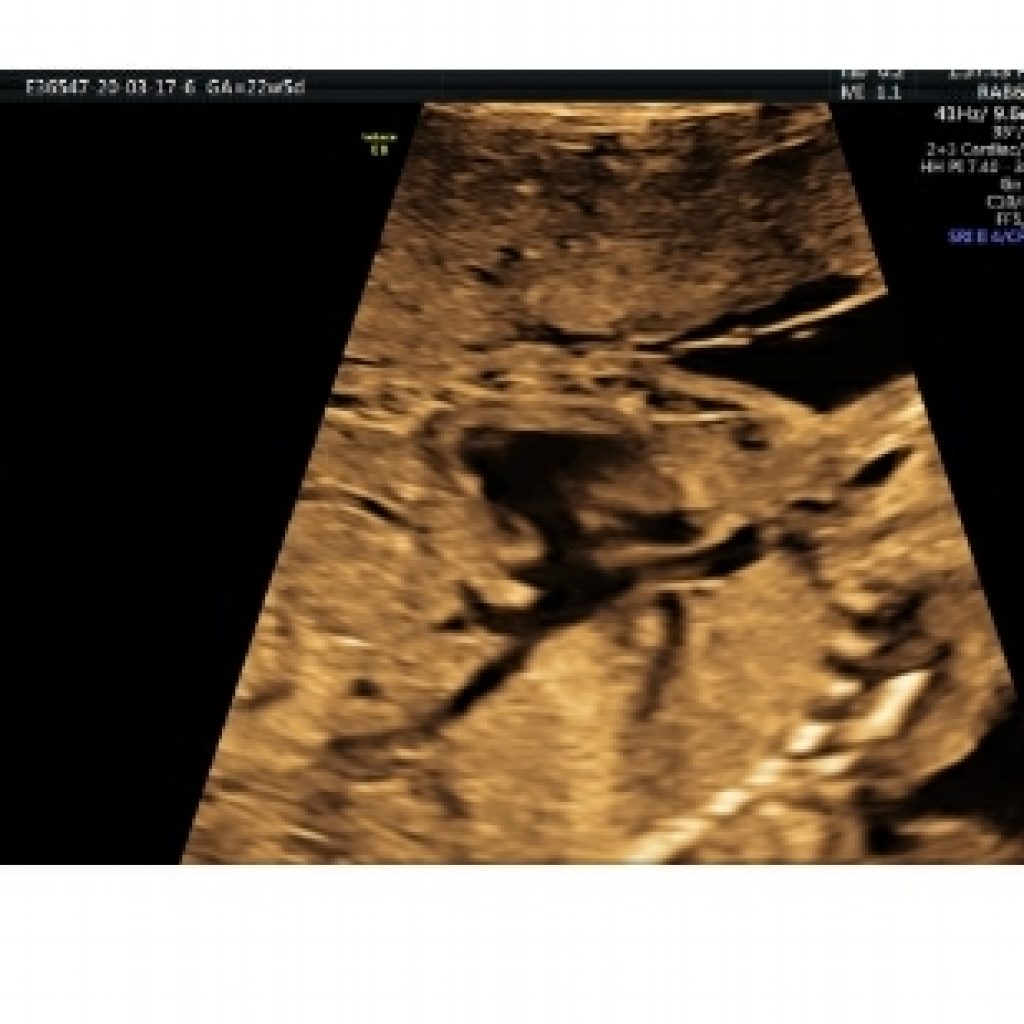
The bicaval view is a parasagittal view showing the SVC and IVC entering the right atrium.(fig 19).The SVC and IVC should be similar in size, and it is important to follow the IVC into the liver for some distance to make sure that it is not interrupted as may be seen in azygos continuation of the IVC, which is associated with heterotaxy syndromes. The right hepatic vein is frequently visible in the liver on this view as well
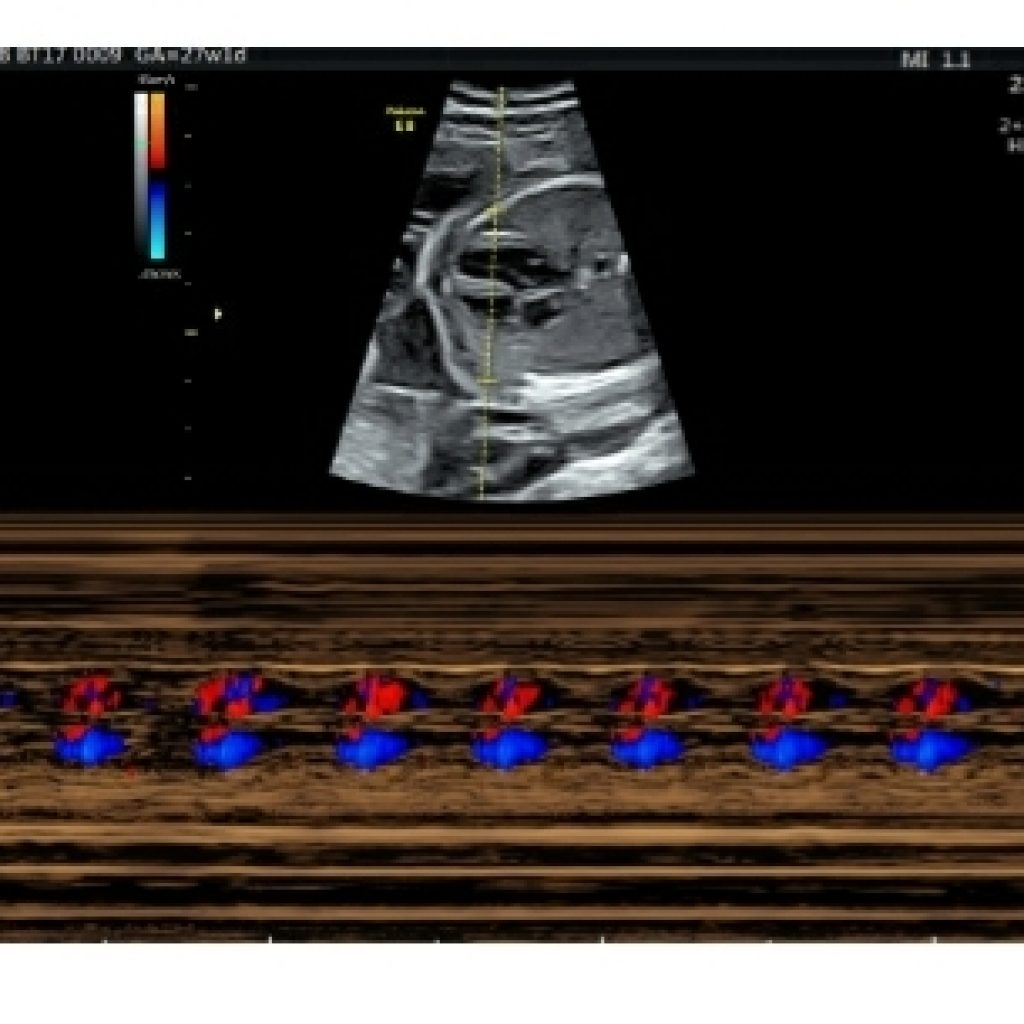
Rhythm
A normal fetal heart rate at midgestation is 120 to 180 beats per minute. If bradycardia or tachycardia is documented, or if the rhythm is noted to be irregular, a detailed assessment of atrial and ventricular contractions should be performed (fig 20a,20b)
The identification of atrioventricular valve inflow serves as a proxy for atrial systole. Ventricular systole can be identified with M-mode of the ventricular free wall or aortic valve or again, using pulse Doppler interrogation of the outflow tract or tissue Doppler of the ventricular myocardium. Doppler flow in the outflow tract serves as a proxy for ventricular systole
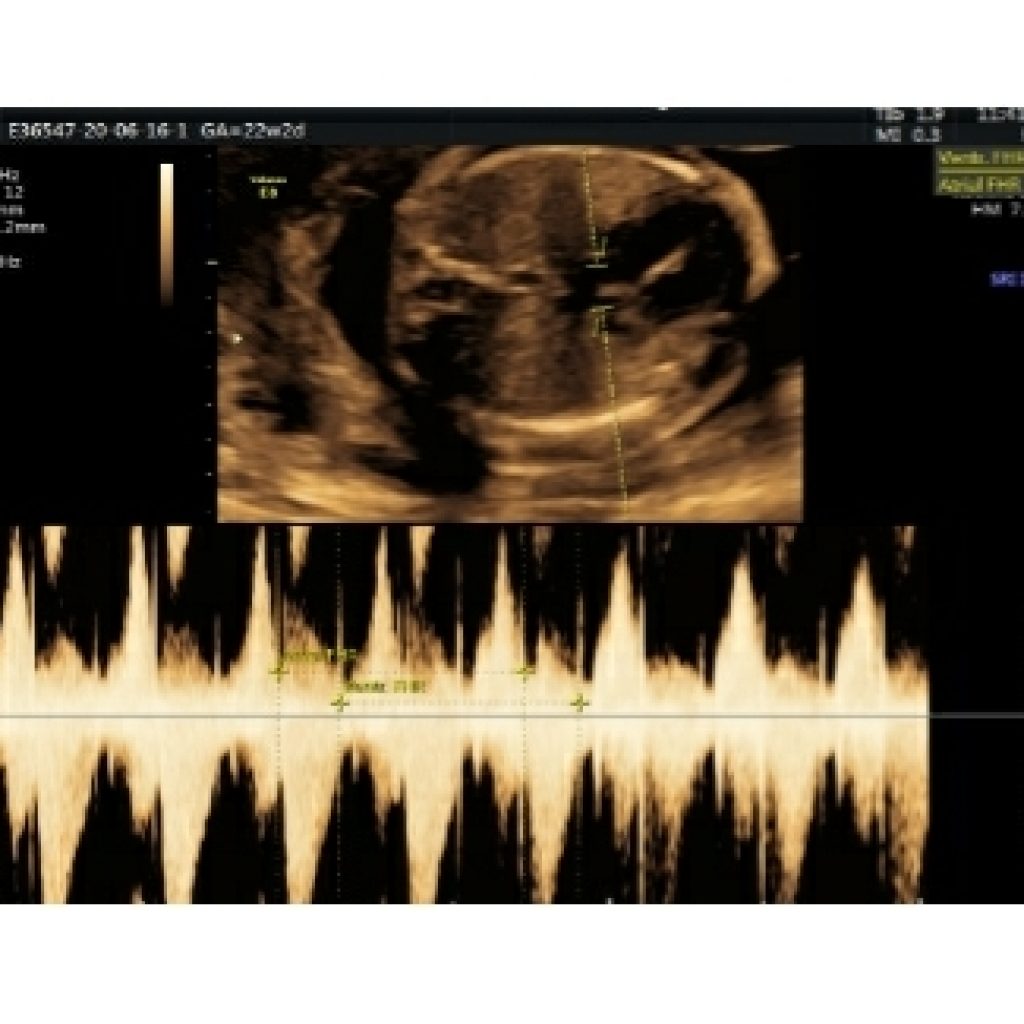
Measurement of the time interval between two successive beats allows calculation of rate. Simultaneous interrogation of left ventricular inflow and outflow (mitral valve and LVOT allows assessment of atrioventricular conduction and the “mechanical” PR interval (fig 21).
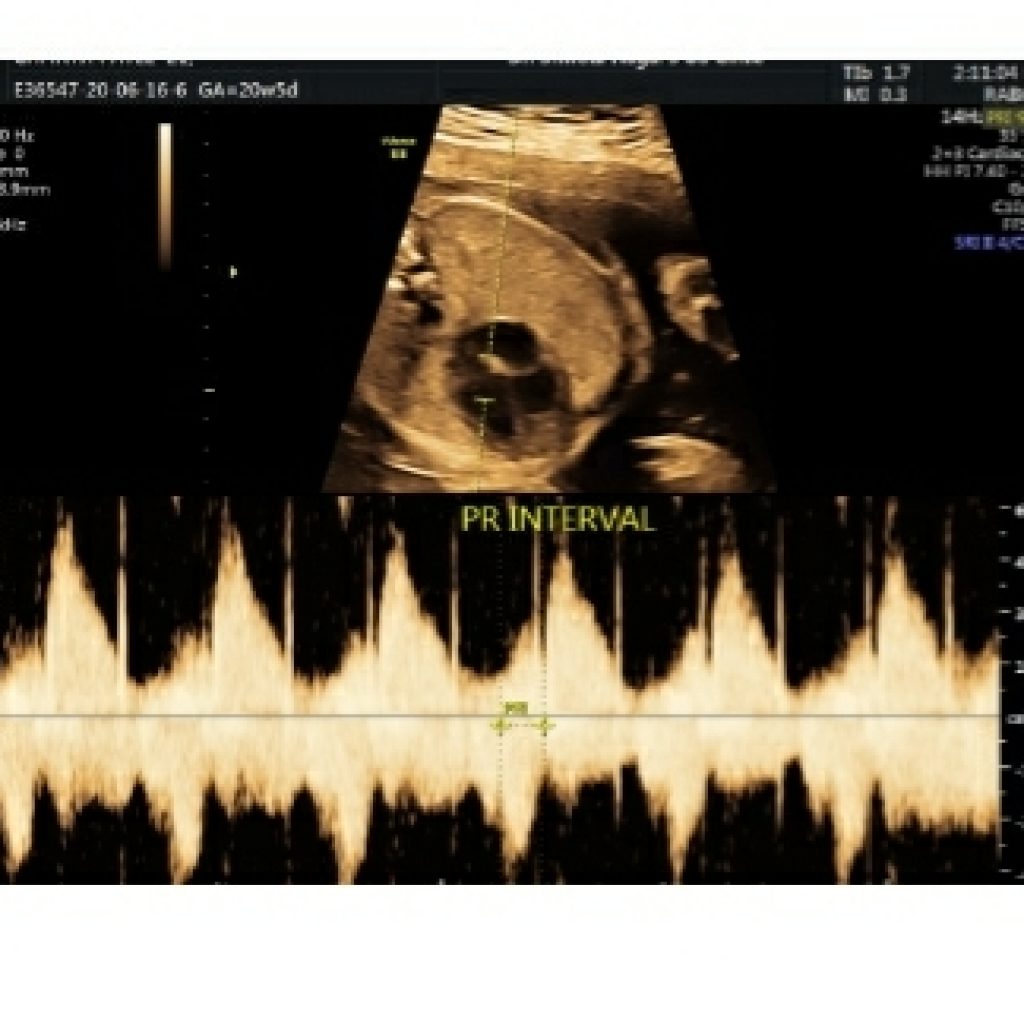
Colour flow and Doppler measurements
Colour flow
• Systemic veins (including superior and inferior venae cavae and ductus venosus)
• Pulmonary veins (at least two: one right vein and one left vein)
• Atrial septum and foramen ovale
• Atrioventricular valves Ventricular septum
• Semilunar valves
• Ductal arch
• Aortic arch
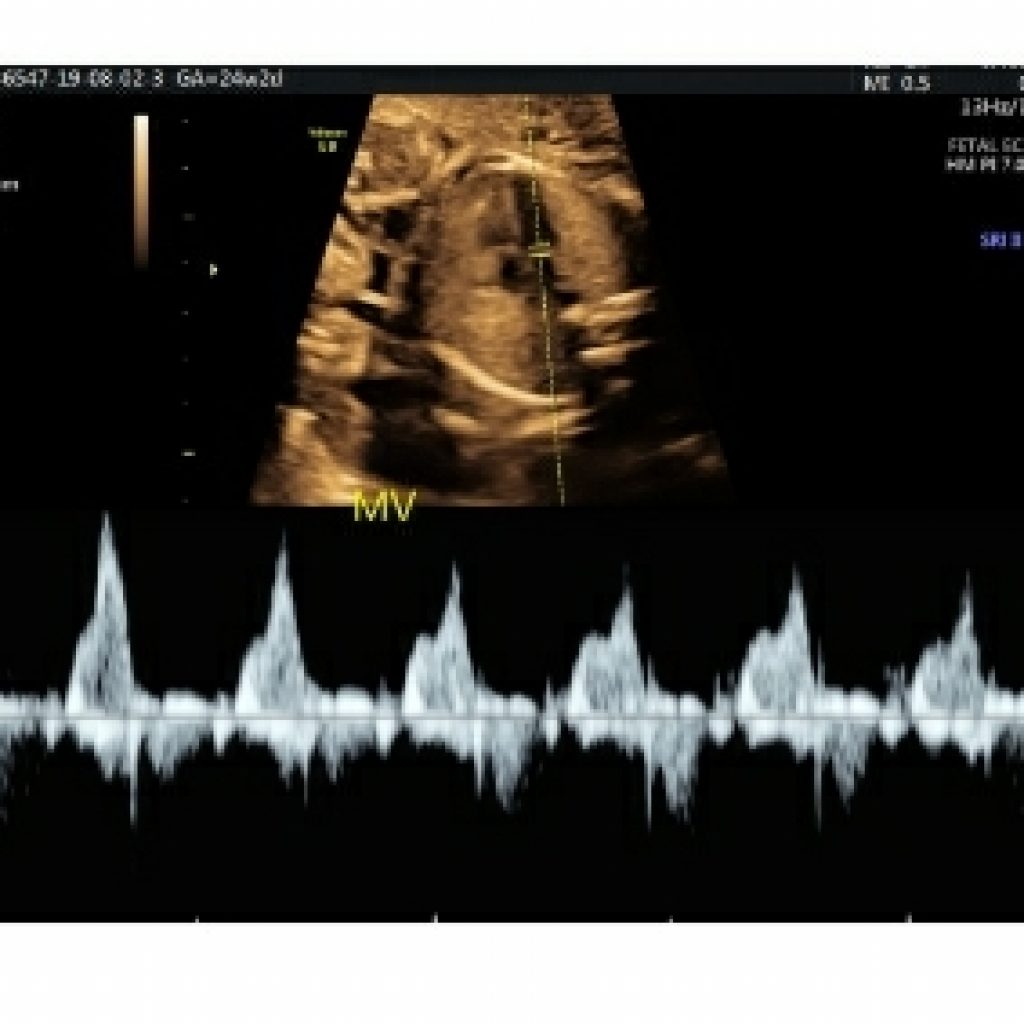
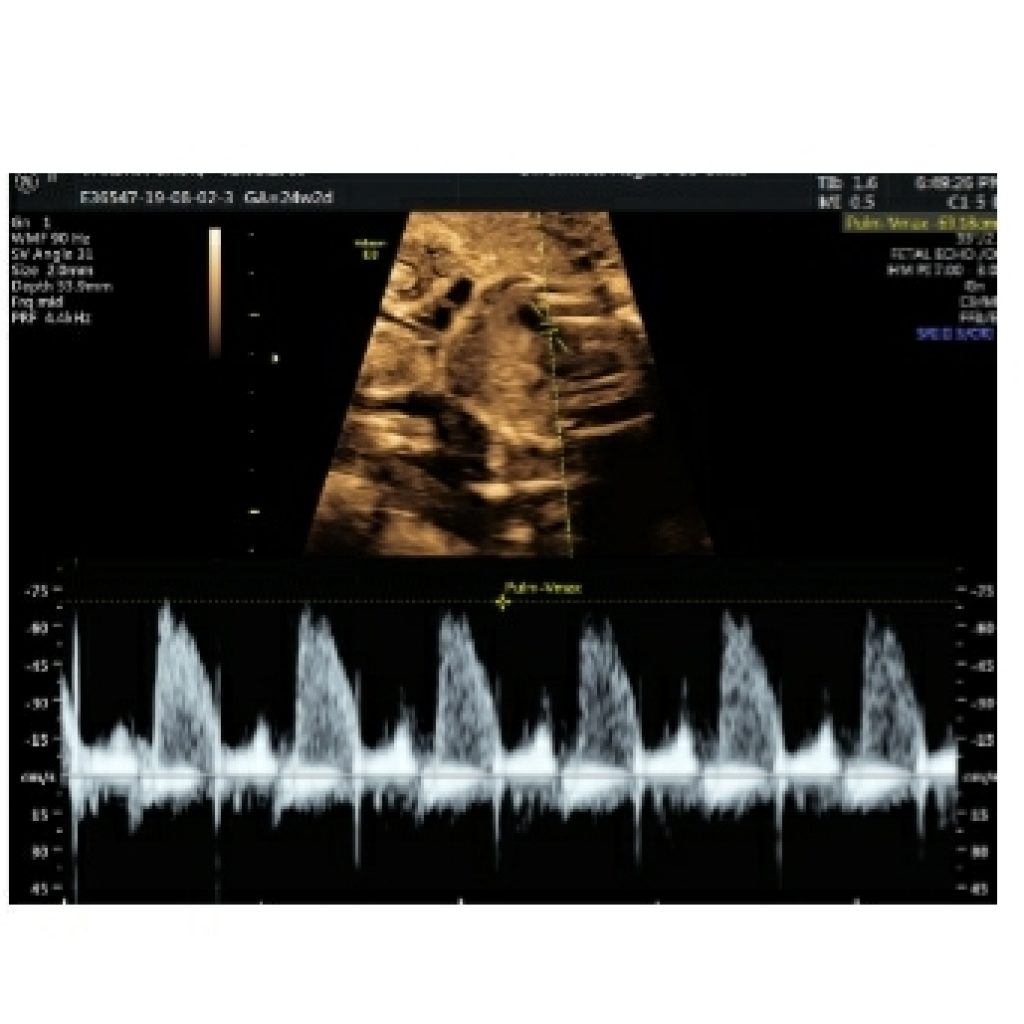
Pulsed Doppler Ultrasound (Required)
Pulsed-wave Doppler ultrasound should be used to evaluate the following:
• Right and left atrioventricular valves
• Right and left semilunar valves
• Pulmonary veins (at least two: one right vein and one left vein)
• Ductus venosus
• Suspected structural or flow abnormality on color Doppler imaging.
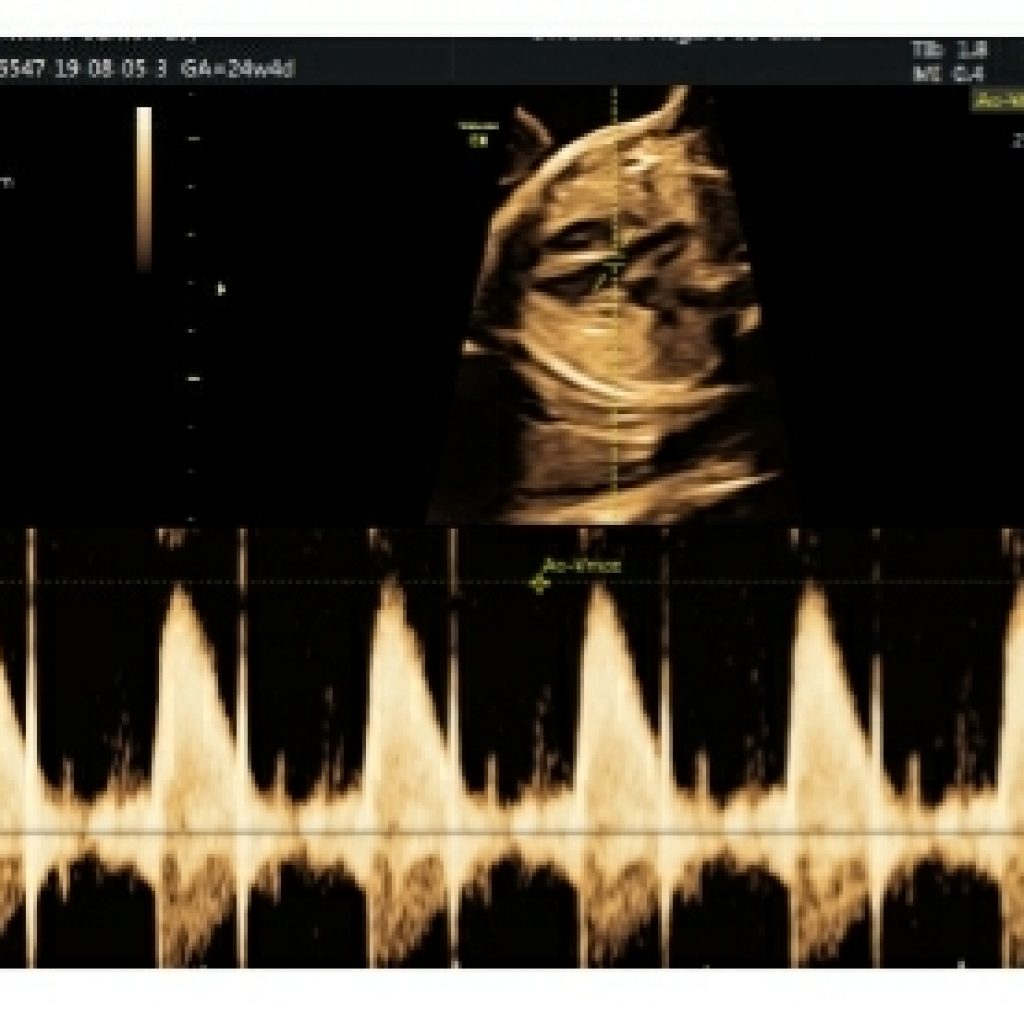
Pulsed-wave Doppler ultrasound may also be clinically relevant for evaluating the ductus arteriosus, systemic veins (eg, superior vena cava, inferior vena cava, and hepatic veins), aortic arch at the isthmus, branch pulmonary arteries, middle cerebral artery, and umbilical artery or vein.(fig 22)
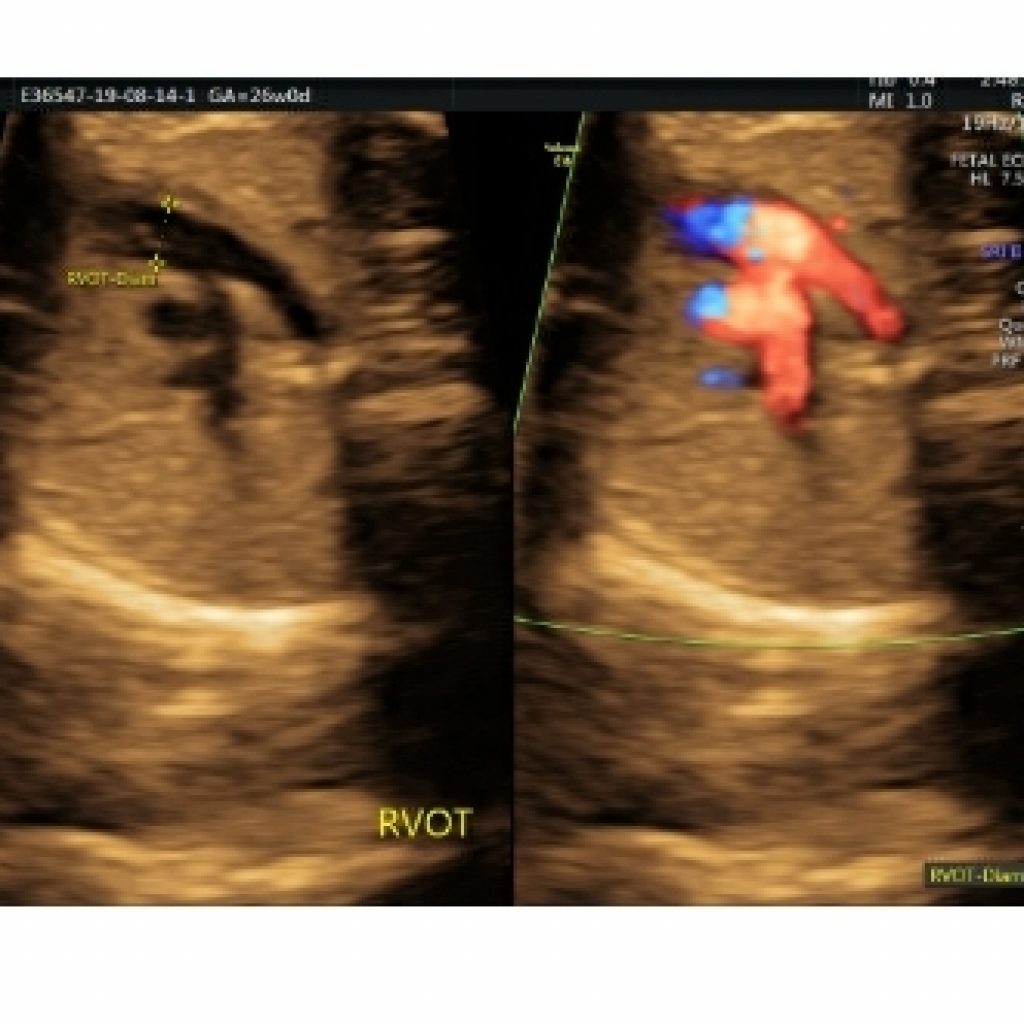
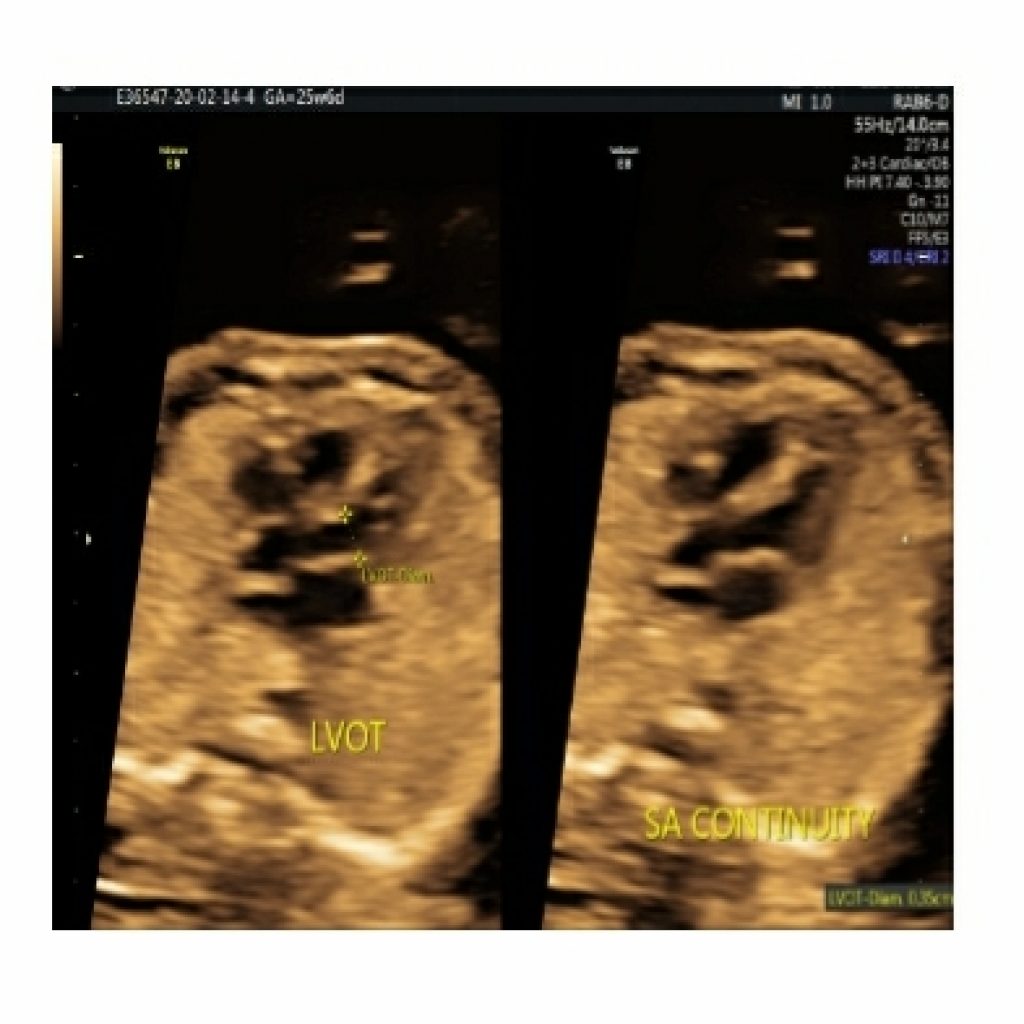
Fig 22a,b– Transverse axial fetal thoracic view split gray scale and colour Doppler images demonstrates measurement caliper placement measuring diameter of LVOT & RVOT
Summary of steps
• Define laterality
• Image the 4-chamber view
• Image the abdominal situs
• Image the left ventricular outflow tract (aortic origin)
• Image the pulmonary artery and duct (3VV)
• Image the transverse aortic arch
• Image the left ventricular outflow tract
• Image the arch and duct simultaneously
• (Image the longitudinal views of heart – optional)
A standard fetal echocardiogram consists of several specific views which can be obtained to optimize visualization of different structures and anomalies. They include:
Basic views
• abdominal situs view / transverse view of abdomen
• four chamber view
• left ventricular outflow tract view (or a five-chamber view)
• right ventricular outflow tract view / three-vessel view (3VV)
• three vessel and trachea view (3VT)
Additional views
• short axis view of the great vessels
• aortic arch view
• ductal arch view
• IVC and SVC
• long axis left ventricular outflow tract view
• color Doppler over the interventricular septum
Check list
Abdominal situs Visceral/abdominal situs:
• Position of the stomach, portal vein, descending aorta, and inferior vena cava in the axial view of
the abdomen
Cardiac situs
Cardiac axis : normal/left /right axis deviation
Cardiac apex –left/right/ mesocardia.
Cardiac apex position and cardiac axis in the axial view of the chest
Four-chamber view, including pulmonary veins.
• Atria:
Atrial anatomy (including the septum, foramen ovale, and septum primum)
• Ventricles:
right ventricle-moderator band .
left ventricle –elongated and deep
• Right and left ventricular anatomy (including the septum)
Position-apex formed by left ventricle
-Right ventricle close to chest wall
• Atrioventricular connections (including offsetting of the mitral and tricuspid valves)
Atrioventricular junction: anatomy, size, and function (stenosis or regurgitation) of atrioventricular (eg, mitral and tricuspid valve or common atrioventricular)
• Relative and absolute sizes (z scores/percentiles)
• Systolic function
• Left ventricular outflow tract
• Right ventricular outflow tract
• Ventriculoarterial connections.
•Great arteries (aorta, main and branch pulmonary arteries, and ductus arteriosus):(fig 23 a,b)
•Valves of semilunar (eg, aortic and pulmonary or truncal) valves, including assessments of both the subpulmonary and subaortic regions.
• Vessel size, patency, and flow (both velocity and direction)
•Relative and absolute sizes of the aortic isthmus and ductus arteriosus
•Branch pulmonary artery bifurcation
•Main pulmonary artery diameter
right branch
left branch
• Three-vessel view (including a view with pulmonary artery bifurcation and a more superior view with the ductal arch)
• Position of the transverse aortic arch and ductus arteriosus relative to the trachea normal /left or right .
• 3VT view:area behind the heart no additional vessel apart from aorta
•thymus : thymic –thoracic ratio=>0.45 normal /hypoplasia
Pericardium
• Short-axis views (“low” for ventricles and “high” for outflow tracts)
• Long-axis view (if clinically relevant)
• Aortic arch
• Ductal arch
• Superior and inferior venae cavae
Color Doppler Ultrasound (Required)(24a,b,c,d)
Color Doppler ultrasound should be used to evaluate the following structures for potential flow disturbances:
• Atrial septum and foramen ovale
• Atrioventricular valves
Ventricular septum
• Semilunar valves
• Ductal arch
• Aortic arch
• Systemic veins (including superior and inferior venae cavae and ductus venosus)
• Pulmonary veins (at least two: one right vein and one left vein)
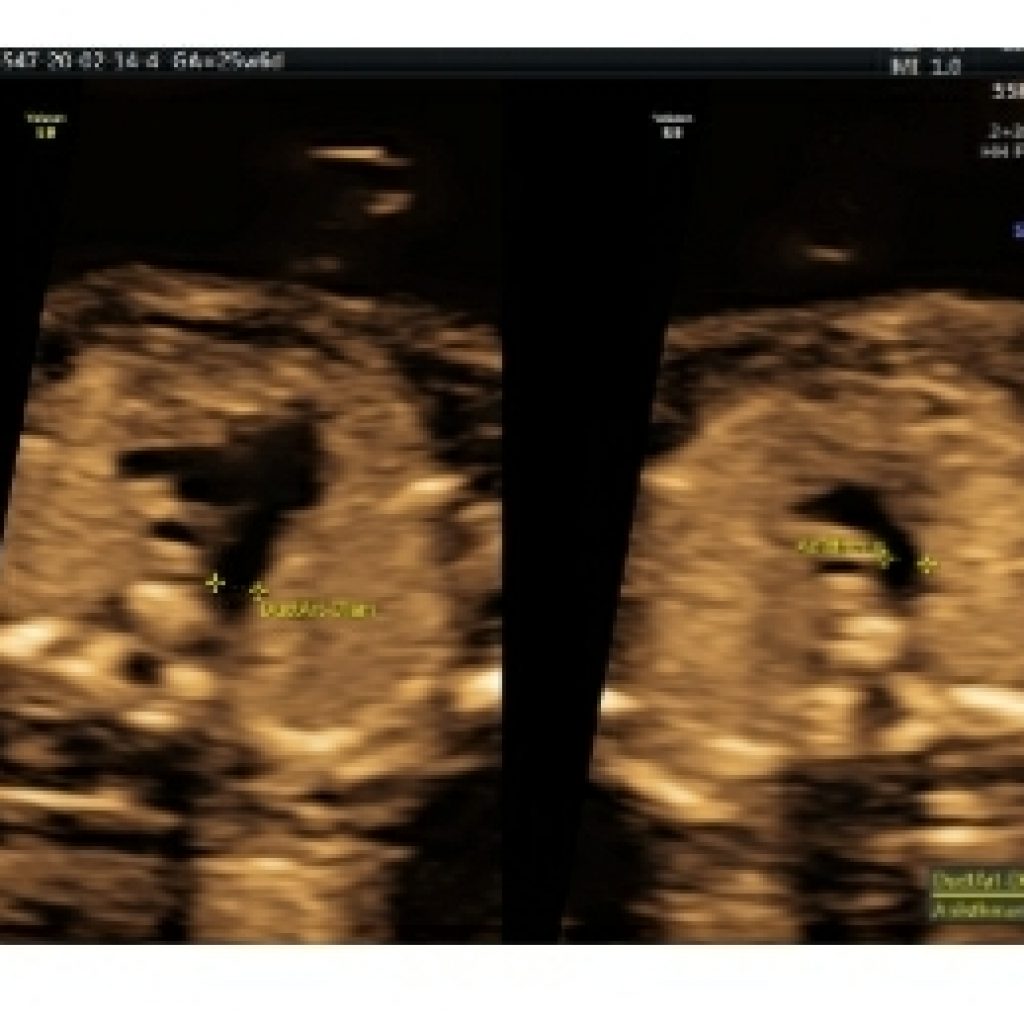
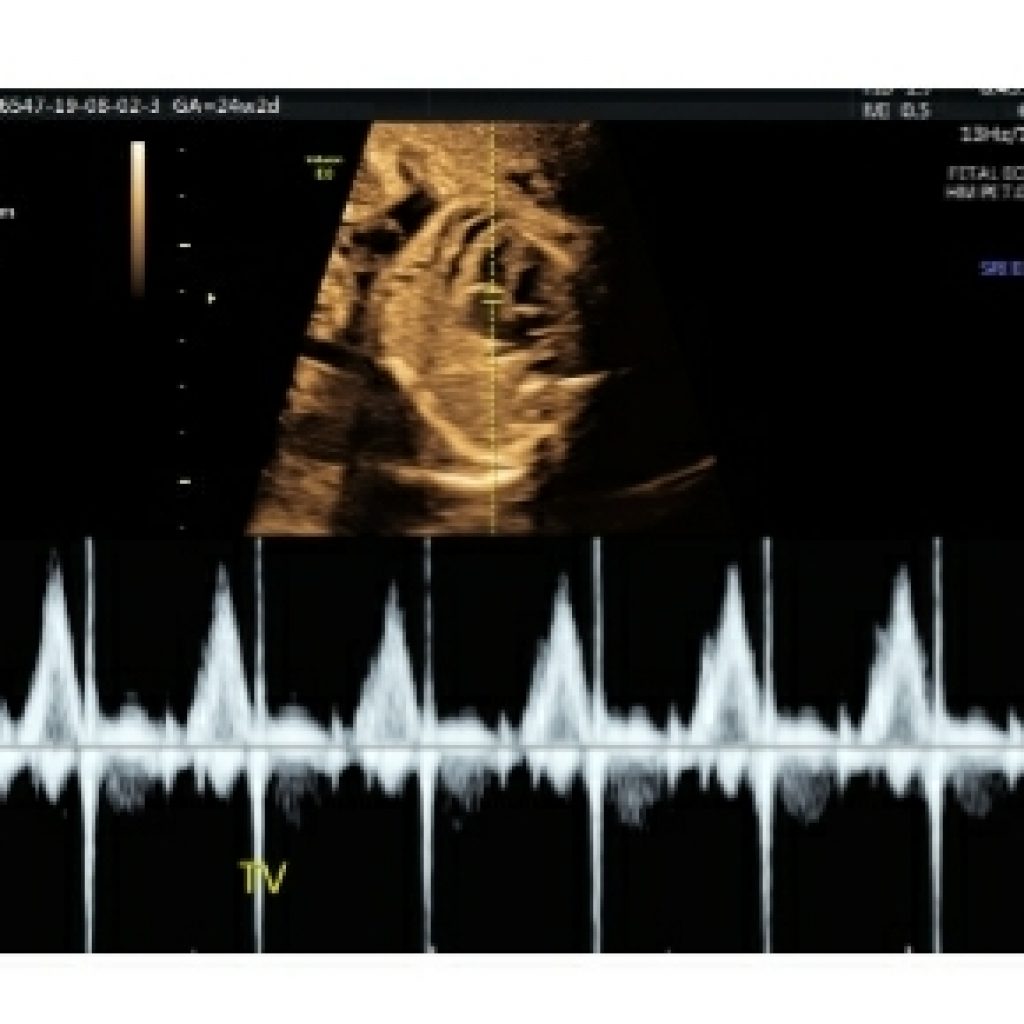
Pulsed Doppler Ultrasound (Required) (fig 25)
Pulsed-wave Doppler ultrasound should be used to evaluate the following:
• Right and left atrioventricular valves
• Right and left semilunar valves
• Pulmonary veins (at least two: one right vein and one left vein)
• Ductus venosus
• Aortic isthmus
• Suspected structural or flow abnormality on color Doppler imaging
Conclusion
CHD may be isolated but it may indicate aneuploidy or a syndrome. When present, aneuploidy and other anomalies determine the prognosis. When isolated, the prognosis is determined by the exact nature of the abnormalities. Mild form of CHD may just require serial monitoring the child over time whereas complex CHD may require counseling ,decision making and options of surgical intervention.
Hence in any fetal ultrasound evaluation any suspicion of CHD should be identified and referred to tertiary center for further dedicated fetal echocardiography by skilled radiologist. A systematic segmental analysis as outlined in this guideline will help in confident determination of normal versus abnormal. Once the type of abnormality is identified a road map can be established through parent counseling for further management.
References
- Dolk H, Loane M, EUROCAT Steering Committee. Congenital Heart Defect in Europe: 2000-2005. Newtownabbey, Northern Ireland: University of Ulster; March 2009. Available from: http://eurocat.bio-medical.co.uk/content/Special-Report.pdf
- Bernier PL, Stefanescu A, Samoukovic G, Tchervenkov CI. The challenge of congenital heart disease worldwide: epidemiologic and demographic facts. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2010;13:26-34
- Hoffman JIE. The global burden of congenital heart disease. Cardiovascular J Africa. 2013;24:141-5
- Hoffman JIE. Incidence of congenital heart disease: I. Postnatal incidence. Pediatr Cardiol. 1995;16:103-13
- Hoffman JI, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. 2002;39:1890-900
- van der Linde D, Konings EM, Slager MA, Witsenburg M, illem Helbing A, Johanna JM, et al. Birth prevalence of congenital heart disease worldwide. A systematic review and meta-analysis. J Am Coll Cardiol. 2011;58:2241-7
- Kothari SS, Gupta SK. Prevalence of congenital heart disease. Prevalence of congenital heart disease. Indian J Pediatr. 2013;80:337-9
- Saxena A. Congenital Heart Disease in India: A Status Report. Indian Pediatr 2018;55:1075-1082
- Khalil A, Aggarwal R, Thirupuram S, Arora R. Incidence of congenital heart disease among hospital live births in India. Indian Pediatr. 1994;31:519-27
- Vaidyanathan B, Sathish G, Mohanan ST, Sundaram KR, Warrier KK, Kumar RK. Clinical screening for congenital heart disease at birth: A prospective study in a community hospital in Kerala. Indian Pediatr.2011;48:25-30
- Sawant SP, Amin AS, Bhat M. Prevalence, pattern and outcome of congenital heart disease in Bhabha atomic research centre hospital. Indian J Pediatr. 2013;80:286-91
- Saxena A, Mehta A, Sharma M, Salhan S, Kalaivani M, Ramakrishnan S, et al. Birth prevalence of congenital heart disease: A cross sectional observational study from North India. Ann Pediatr Cardiol. 2016;9:205-9
- Fetal Cardiac US: Techniques and Normal Anatomy Correlated with Adult CT and MR Imaging Neel Patel, Evan Narasimhan, Anne Kennedy , RadioGraphics > Vol. 37, No. 4
- AIUM Practice Parameter for the Performance of Fetal Echocardiography,American Institute of Ultrasound in Medicine , JUltrasoundMed2020;39:E5–E16 | 0278-4297 .
- ISUOG Practice Guidelines (updated): sonographic screening examination of the fetal heart Ultrasound Obstet Gynecol 2013; 41: 348–359.
- American Society of Echocardiography Guidelines and Standards for Performance of the Fetal Echocardiogram , Journal of the American Society of Echocardiography Volume 17 Number 7.
- Chaubal NG, Chaubal J. Fetal echocardiography. Indian J Radiol Imaging 2009;19:60-8
- Karande A, Nagar S. Fetal echocardiography: A systematic approach. J Indian Acad Echocardiogr Cardiovasc Imaging 2017;1:47-54
