Authors: Rijo M Choorakuttil, Vanaj Mathur, Lalit K Sharma, Anjali Gupta, Shilpa Satarkar, Renu Sharma, Akanksha Baghel, Neelam Jain, Bavaharan R, Devarajan P, Ramesh S Shenoy, M.R. Balachandran Nair, Praveen K Nirmalan for Team Samrakshan
Author Affiliations:
- Rijo M Choorakuttil, National Coordinator for Samrakshan IRIA, AMMA Center for Diagnosis and Preventive Medicine, Kochi, Kerala, India
- Vanaj Mathur, Dr.Mathur’s Ultrasound,Colour Doppler & Digital X-ray Centre, 2/2, Swadeshi Bima Nagar Civil Lines, Agra-282002, UP
- Lalit K Sharma, Raj Sonography & X- Ray Clinic, Baiju Choraha, Nayapura, Guna, Madhya Pradesh, India
- Anjali Gupta, Anjali Ultrasound and Colour Doppler centre, 2nd floor, Shanti Madhuban Plaza, Delhi Gate, Agra, Uttar Pradesh, India
- Shilpa R Satarkar, Antarang Sonography and Colour Doppler Center, Satarkar Hospital, Plot 20. Tilaknagar, Aurangabad, Maharashtra, India
- Renu Sharma, Dr Renu’s Diagnostic Center,E 6 Basant Vihar, Sikar, Rajasthan, India
- Akanksha Baghel, Baghel Sonography Center. Front of Janpat Office, near District Hospital, Harda, Madhya Pradesh, India
- Neelam Jain, Jain Ultrasound centre, C-112, B block, Dispensary road Sonari, Jamshedpur Jharkhand, India
- Bavaharan R, Fetocare Magnum Imaging and Diagnostics, Trichy, Tamil Nadu, India
- Devarajan P, Nethra Scans and Genetic Clinic, Tiruppur, Tamil Nadu, India
- Ramesh S Shenoy, Consultant Radiologist, Lisie Hospital, Ernakulam, Kerala, India
- M.R. Balachandran Nair, Department of Radiology, Jubilee Mission Hospital, Thrissur, Kerala, India
- Praveen K Nirmalan, Chief Research Mentor, AMMA ERF, AMMA Center for Diagnosis and Preventive Medicine, Kochi, Kerala, India
Running Title: Outcomes of Childbirth
Corresponding Author: Rijo M Choorakuttil, National Coordinator for Samrakshan IRIA, AMMA Center for Diagnosis and Preventive Medicine, Kochi, Kerala, India.
E mail: samrakshaniria@gmail.com
Key words: Pregnancy, Fetal Radiology, Colour Doppler, Ultrasound, Outcomes, Samrakshan, India
Abstract
Aim: To determine magnitude of perinatal outcomes and characteristics as part of the baseline data of Samrakshan India
Methods: Details of child birth of women screened in the Samrakshan program were stored in an online database without individual patient identifiers. The incidence of abnormal Doppler studies, Pre eclampsia and fetal growth restriction, gestational age at delivery, estimated fetal weight and birth weights, neonatal mortality and still births were collected.
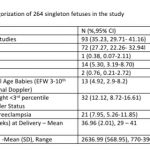
Results: The data of 264 child births among women screened as part of Samrakshan between July 2019 and February 2020 was analyzed. An abnormal Doppler study in the 3rd trimester was present in 93 (35.23%, 95% CI: 29.71, 41.16) of the 264 women. Twenty-six (9.85%, 95% CI: 6.81, 14.04) women had developed pre-eclampsia during the course of pregnancy. 78 (29.54%, 95% CI: 24.37, 35.31) women had a preterm birth (<37 weeks). 56.94% of the fetuses with stage 1 FGR had childbirth at or more than 37 gestation weeks with a mean birthweight of 2603.96 (393.24) grams and 27 of these 41 fetuses attained a birthweight greater than 2500 grams. The neonatal mortality rate was 15.32 per 1000 live births and perinatal mortality rate was 26.52 per 1000 pregnancies.
Conclusion: The preliminary baseline data from Samrakshan shows the potential for reduction in perinatal mortality through a systematic, concerted, liaised approach that involves trimester specific screening protocols integrating colour Doppler ultrasound studies.
Introduction
Samrakshan, the national program of the Indian Radiological and Imaging Association (IRIA) aims to reduce perinatal mortality in India.[1] The program utilizes an individual competing risk assessment based trimester specific algorithm and protocols integrating colour Doppler ultrasound studies to screen pregnant women and identify women at risk for preterm preeclampsia (PE) and fetal growth restriction (FGR). [2-6] Samrakshan has specific offline and online training modules and CME programs for radiologists and a data-based evidence generation and evaluation process. [1,7] Samrakshan has previously published baseline information from the initial 8 months related to the 2nd and 3rd trimester of pregnancy as part of the evaluation process of the program.[2,3,6] In this manuscript, we report on the childbirth outcomes for the first 8 months of the program. The baseline information will be used to benchmark improvement of perinatal outcome measures in Samrakshan.
Methods
The processes and protocols of Samrakshan have been described previously.[1-3,6] Briefly, each woman screened in Samrakshan is assigned a unique identification number that is used to track further documentation through pregnancy. Clinico-demographic details including maternal age, type of conception, parity, height and weight, past obstetric history, and presence of co-morbidity was collected from each woman. Systolic and diastolic blood pressures were measured in the seated position [4] to derive a mean arterial blood pressure (MAP). Routine ultrasound examinations were performed in each trimester and included measures of amniotic fluid using the single deepest vertical pocket in the 2nd trimester and amniotic fluid index in the 3rd trimester [8,9], and measures of placental thickness. The estimated fetal weight (EFW) was derived using the Hadlock III formula that included fetal abdominal circumference, head circumference and femur length. [10] Additionally, trimester specific colour Doppler studies were performed using standard measurement guidelines. Abnormal Doppler studies included one or more of mean uterine artery pulsatility index (PI) >95th percentile, umbilical artery PI >95th percentile, middle cerebral artery (MCA) Doppler PI <5th percentile, a Cerebro-Placental Ratio (CPR) <5th percentile, absent or reversed end diastolic flow velocity, and an abnormal Ductus Venosus.[11-13]
Fetal Growth was categorized as Stages 1 -4, no fetal growth restriction (FGR) or small for gestational age (SGA) and managed based on the protocol described by Figueras at al. [5] A SGA fetus was defined as a fetus with EFW 3-10th percentile and a normal Doppler study.[5] Childbirth outcomes of interest included preterm births, birthweight, neonatal mortality and still births.
Data was entered and submitted as online forms that were stored in a secure, password protected database. Data was exported to MS Excel for data cleaning and primary analysis and additional analyses were performed using the open source Open Epi program. The frequency distribution of categorical variables were expressed as proportions and 95% confidence intervals around the point estimates. Continuous variables were expressed as mean and standard deviation and compared using a parametric students t-test. A p value <0.05 was considered statistically significant. Neonatal mortality rate was defined as the number of neonatal deaths among live births and expressed as per 1,000 live births. Perinatal mortality rate was defined as the number of perinatal deaths (including still births) among pregnancies and expressed as per 1,000 pregnancies.
Results
The data of 264 child births, for whom outcomes forms were received, among women screened as part of Samrakshan between July 2019 and February 2020 was analyzed. The mean age (SD) of these women was 27.43 (4.88) years. Twenty-six (9.85%, 95% CI: 6.81, 14.04) women were 35 years or older and 54 (20.45%, 95% CI: 16.03, 25.73) women were between 30 and 35 years. The majority of women had a spontaneous or natural conception (n=262) and 132 (50.00%, 95% CI: 43.98, 56.02) women were nulliparous.
An abnormal Doppler study in the 3rd trimester was present in 93 (35.23%, 95% CI: 29.71, 41.16) of the 264 women with documented childbirth outcomes (see Table-1). These included 39 (14.77%, 95% CI: 11, 19.56) mean uterine artery PI >95th percentile, 29 (10.98%, 95% CI: 7.76, 15.33) umbilical artery PI >95th percentile, 37 ( 14.01%, 95% CI: 10.34, 18.72) MCA PI <5th percentile and 57 (21.59%, 95% CI: 17.05, 26.94%) CPR <5th percentile. The mean (SD) gestational age at delivery (36.67 (2.52) weeks) for those with abnormal Doppler studies did not differ significantly (student’s t-test p value 0.15) from those with a normal Doppler study (37.08 (1.65) weeks).
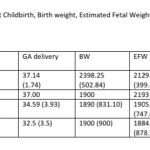
The distribution of gestational age at delivery, birth weight and EFW at staging of FGR is presented in Table-2. Forty-one (56.94%, 95% CI: 45.44, 67.74) of the fetuses with stage 1 FGR had childbirth at or more than 37 gestation weeks. The mean birthweight of this subset ( stage 1 FGR, term delivery) was 2603.96 (393.24) grams. Twenty seven of these 41 fetuses attained a birthweight greater than 2500 grams. There was a significant improvement in birth weight compared to EFW in fetuses with Stage 1 FGR (p=0.0006). The mean (SD) interval between gestational age at staging of FGR in the 3rd trimester to childbirth was 2.02 (1.73) weeks (range <1week to 8 weeks) for stage 1 FGR fetuses in this series. The birth weight and EFW did not differ significantly for stage 3 (p=0.96) and stage 4 FGR fetuses (p=-0.98). The EFW and birth weight was not compared for stage 2 FGR as there was only 1 fetus in this subgroup.

Outcomes were available for 13 fetuses identified as SGA and were advised routine follow up instead of close surveillance. The mean gestational age at delivery for this subgroup was 36.17 (2.24) weeks and the mean birth weight was 2298.46 (435.30) grams. There was no neonatal mortality among fetuses identified as SGA in this series.
The mean gestational age at delivery was 36.13 (1.25) weeks and birth weight 1926.37 (302.28) grams for fetuses (n=32) identified as EFW <3rd percentile in the 3rd trimester. Three of these 32 fetuses had a neonatal death.
Outcomes were available for 42 fetuses with an EFW 10-50th percentile (AGA) and abnormal Doppler study. The mean gestational age at delivery was 37.28 (2.04) weeks and mean birth weight was 2623.48 (489.25) grams for these 42 fetuses. One of these 42 fetuses had an umbilical artery PI >95th percentile, MCA <5th percentile, CPR <5th percentile and DV >95th percentile. This fetus had an emergency delivery at 30.3 weeks but succumbed in the early neonatal period.
Twenty-six (9.85%, 95% CI: 6.81, 14.04) women had developed pre-eclampsia during the course of pregnancy. This included 21 (7.95%, 95% CI:5.26, 11.85) women who developed preterm PE <37 gestation weeks. 5 of these 21 women with maternal preterm PE developed PE before 34 gestation weeks. The mean gestational age for women with PE was 34.07 (3.17) weeks and mean birth weight was 1880 (649.38) grams.
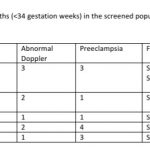
78 (29.54%, 95% CI: 24.37, 35.31) women had a preterm birth (<37 weeks). This included 32 pregnancies with abnormal Doppler study and 20 pregnancies with preterm PE. 15 of the 78 preterm births (19.23% of preterm births or 5.68% of total childbirths) were earlier than 34 weeks (See Table-3). Twelve (80%) of these 15 women had preterm PE. Childbirth occurred at 40 weeks or more in 15 pregnancies (5.68%) that included 4 fetuses categorized as stage 1 FGR and 2 fetuses categorized as stage 3 FGR.
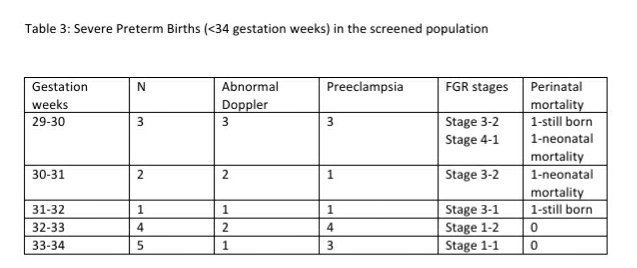
Overall, there were 4 neonatal deaths in this series of 264 fetuses. All 4 these pregnancies with neonatal mortality had an abnormal Doppler study in the antenatal period, 1 of the 4 mothers had preterm PE and 3 of the 4 fetuses had an EFW <3rd percentile, and one fetus had an EFW 10-50th percentile respectively. All 4 fetuses had a stage 3 FGR and were delivered preterm at gestational ages of 29, 30.3, 34 and 35 weeks. The neonatal mortality rate was 15.32 per 1000 live births.
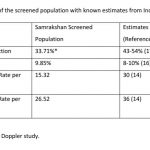
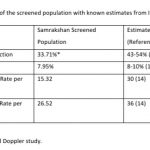
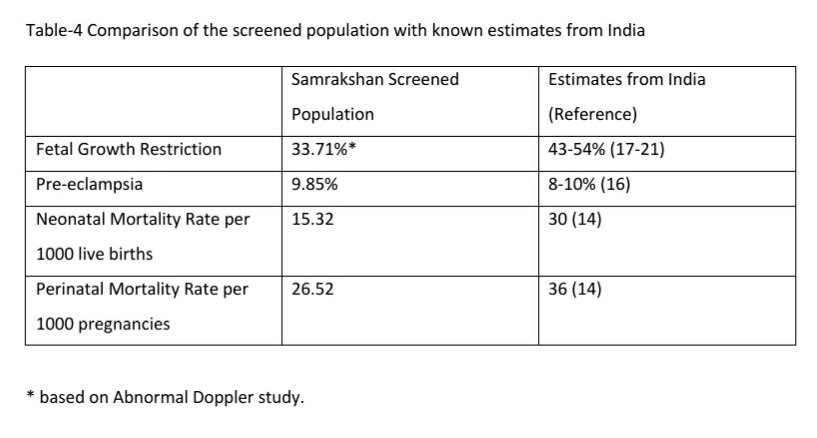
There were 3 still births in this series. Maternal preterm PE was present in 2 still births with Doppler findings consistent with Stage 3 and Stage 4 FGR and birth at less than 34 weeks. One of these still births had associated major congenital abnormalities. One of the still births occurred at 39 weeks in a woman with gestational diabetes diagnosed elsewhere. The first visit in the program for this woman was at 34 weeks and the fetus had EFW 10-50th percentile and a normal Doppler study at this visit. The woman did not return for the recommended follow up at 36 weeks and presented at the 39th week with loss of fetal movements of 3 days duration and ultrasound exam confirmed intrauterine fetal demise. The perinatal mortality rate was 26.52 per 1000 pregnancies. Table -4 compares the magnitude of FGR, PE and Perinatal mortality in the screened population with known estimates from India.
Discussion
The primary aim of Samrakshan is to utilize a synergistic and holistic approach that builds on the skill sets and expertise of fetal radiologists in India to complement and supplement existing efforts to address perinatal mortality in India.[1] The primary outcome of interest is the perinatal mortality rate. [1] Factors that have a major impact on perinatal mortality rates include FGR, PE and preterm births and form the core secondary outcome measures of interest relevant to childbirth. [1]
It is important to have a benchmark estimate to measure the progress and impact of the program. Perinatal Statistics in India vary by state and even by districts within states based on diversity of geographical, economic, infrastructure, social and cultural determinants. The statistics also differ based on the definitions as well as methodological approaches used to estimate magnitude. Currently, an estimated 8-10% of pregnant women may develop pre eclampsia in India, intrauterine growth restriction estimates have reduced from 54% of pregnancies in 1995 to 43% in 2014, low birth weight in about 26% of pregnancies and a combination of intrauterine growth restriction and low birth weight is present in an estimated 21% of pregnancies. [14-21] The incidence rate of IUGR is consistently higher than that of IUGR-LBW in all data sets by a mean difference of about 15%.[22] The preterm birth rate for India based on data from 2014 was reported as 13.6% (95% CI: 11.1-16.1); nearly one fourth of the global preterm births are in India.[23] The trends for preterm births, however, show an increasing trend. There are several reasons for the increasing trend in preterm births. [23] These include better measurement and reporting, increases in the population of pregnant women with “older maternal age”, increase in coexisting chronic or lifestyle maternal health problems such as diabetes and high blood pressure, increased prevalence of infertility treatments leading to increased rates of multiple pregnancies, changes in obstetric practices such as more caesarean births before term especially with increased availability and access to quality neonatal intensive care services.
From a Samrakshan program perspective, we decided that it is important have an internal program baseline benchmark for comparison of longitudinal progress besides comparisons with the external nationally available data on indicators of interest. The baseline data will also allow us to determine, to a certain extent, the representativeness of the Samrakshan screened population and to identify areas for focused improvements through the program.
The incidence of pre eclampsia in the screened population was similar to the national reported estimates of prevalence of preeclampsia. However, the incidence of FGR was significantly lower in the screened population. This difference can be attributed to the change in definition with FGR defined primarily on an abnormal Doppler study while the national estimates were based on a percentile (<10th percentile weight) criteria. The incidence of abnormal Doppler studies and FGR is still high with approximately one third of pregnant women having an abnormal Doppler study and/or FGR. Nearly 40% of pregnant women in India need closer surveillance if we consider the magnitude of PE as well. Samrakshan has initiated a 1st trimester screening protocol with the recommendation of low dose aspirin 150 mg at bedtime those found at high risk for preterm PE and FGR. [23] Based on the baseline magnitude of PE and FGR, approximately 35% of the 1st trimester screened population may need preventative use of low dose aspirin and mostly to prevent FGR than PE. Considering that the aim of screening is to reduce false negatives (as FGR and PE have severe consequences) with a possible resultant increase in false screen positives, an estimated 40-45% of the 1st trimester screened population may need low dose aspirin.
The staging of FGR and the stage based management of FGR give us several important benchmarks. The majority of FGR was stage 1 FGR, a milder category of FGR and most of these could be carried till near term with regular follow up. We do not have enough data, at present, to consider if the increased surveillance is useful but the preliminary evidence suggests that the increased focused surveillance with Doppler study of this group may reduce the incidence of preterm births. 56.94% of the fetuses with stage 1 FGR had childbirth at or more than 37 gestation weeks with a mean birthweight of 2603.96 (393.24) grams and 27 of these 41 fetuses attained a birthweight greater than 2500 grams.
Colour Doppler studies helped to identify a subset of fetuses, among FGR fetuses, at increased risk. Our results show a high perinatal mortality rate in stage 3 and stage 4 FGR fetuses indicating the need for priority childbirth in these stages. We also found that the estimated fetal weight, as determined by Hadlock III, was not significantly different from birthweight in stage 3 and Stage 4 FGR fetuses, where assessment to delivery was less than a week, suggesting that Hadlock III may have good accuracy to estimate fetal weight in these small fetuses. However, the program was not designed specifically to address this question and we need a larger sample size to reach any conclusion on that.
The integration of colour Doppler studies helped to differentiate SGA from FGR babies. We found that perinatal outcomes of SGA fetuses remained good even in the absence of intense surveillance. These fetuses were advised routine 3rd trimester follow up similar to AGA babies, had a mean gestational age at delivery close to term and did not have perinatal mortality.
Abnormal Doppler Studies and perinatal mortality is not restricted to fetuses with EFW <10th percentile; we have previously reported on abnormal Doppler studies in fetuses with EFW 10-50th percentile. The mean gestational age of this subgroup was 37.28 (2.04) weeks and mean birth weight was 2623.48 (489.25) grams indicating good perinatal outcomes. One of these fetuses died in the neonatal period with Doppler findings consistent of Stage 4 FGR and major structural abnormalities. We need a larger sample size of outcomes in this subgroup to determine follow up patterns for this subgroup (EFW 10-50th percentile with abnormal Doppler studies) of fetuses.
Maternal PE remains a major influence on perinatal mortality and preterm births in the screened population. We will be able to better discern the preventative effects of low dose aspirin as the program continues and we get a larger sample size of child births in women that received low dose aspirin in the 1st trimester. Besides the impact on the incidence of preterm PE, low dose aspirin may also influence perinatal mortality, incidence of FGR and preterm births.[24] Approximately one third of the screened population had a preterm birth. This may reduce with Samrakshan as rates of PE and FGR may change with low dose aspirin and obstetric childbirth plans may change based on FGR stages with an improved potential to more objectively determine fetuses that can be carried to term.
Samrakshan is currently operational in 10 states with childbirth outcomes available from 3 north Indian and two South Indian states. Our preliminary data shows the potential for reduction in perinatal mortality through a systematic, concerted, liaised approach that involves fetal radiologists, obstetricians and neonatologists in India.
References
- Choorakuttil RM, Patel H, Bavaharan R, Devarajan P, Kanhirat S, Shenoy RS, Tiwari OP, Sodani RK, Sharma LK, Nirmalan PK. Samrakshan: An Indian radiological and imaging association program to reduce perinatal mortality in India. Indian J Radiol Imaging 2019;29:412-7
- Bavaharan R, Choorakuttil RM, Ahuja B, Gupta A, Sharma LK, Baghel A, et al. Routine 3rd Trimester Colour Doppler Ultrasound in Fetuses with Estimated Fetal Weight 10-50th centiles in India- Preliminary Results from the Samrakshan Program. Journal of Fetal Radiology. Accessed online from http://fetalradiology.in/2020/01/16/routine-3rd-trimester-colour-doppler-ultrasound-in-fetuses-with-estimated-fetal-weight-10-50th-centiles-in-india-preliminary-results-from-the-samrakshan-program/ on February 1,2020
- Anjali G, Renu S, Sharma LK, Akanksha B, Bavaharan R, Shilpa RS, at al. Colour Doppler studies identify more fetuses at risk for compromise in the third trimester: Preliminary results from Samrakshan India. Journal of Fetal Radiology. Accessed online from http://fetalradiology.in/2020/02/09/colour-doppler-studies-identify-more-fetuses-at-risk-for-compromise-in-the-third-trimester-preliminary-results-from-samrakshan-india/ on March 1, 2020
- Poon LC, Zymeri NA, Zamprakou A, Syngelaki A, Nicolaides KH. Protocol for measurement of mean arterial pressure at 11‐13 weeks’ gestation. Fetal Diagn Ther. 2012; 31: 42– 48.
- Figueras F, Gratacós E. Update on the diagnosis and classification of fetal growth restriction and proposal of a stage-based management protocol. Fetal Diagn Ther. 2014;36(2):86–98.
- Neelam Jain, Prashant M Onkar, Sunitha Pradeep, Devarajan P, Anjali Gupta, Lalit K Sharma,at al. 2nd trimester screening to identify pregnancies at risk for preterm preeclampsia and fetal growth restriction: Preliminary results from Samrakshan India. Journal of Fetal Radiology. Accessed online from http://fetalradiology.in/2020/02/24/2nd-trimester-screening-to-identify-pregnancies-at-risk-for-preterm-preeclampsia-and-fetal-growth-restriction-preliminary-results-from-samrakshan-india/ on March 1, 2020
- Ramesh Shenoy, Rijo M Choorakuttil, Rajalingam Bavaharan, Palanisamy Devarajan, Praveen K Nirmalan. Mobile Learning as an Integral Part of Samrakshan IRIA national program. Journal of Fetal Radiology. Accessed online at http://fetalradiology.in/2019/11/25/mobile-learning-as-an-integral-part-of-samrakshan-iria-national-program/ on March 1, 2020
- Williams K. Amniotic fluid assessment. Obstet Gynecol Surv 1993; 48: 795– 800.
- Moise KJ, Jr. Toward consistent terminology: assessment and reporting of amniotic fluid volume. Semin Perinatol 2013; 37: 370– 374.
- Hadlock FP, Harrist RB, Sharman RS, Deter RL, Park SK. Estimation of fetal weight with the use of head, body, and femur measurements: a prospective study. Am J Obstet Gynecol. 1985;151:333–7.
- Sotiriadis A, Hernandez‐Andrade E, da Silva Costa F, et al. ISUOG Practice Guidelines: Role of ultrasound in screening for and follow‐up of pre‐eclampsia. Ultrasound Obstet Gynecol. 2019; 53: 7– 22.
- Yu CK, Smith GC, Papageorghiou AT, Cacho AM, Nicolaides KH, Fetal Medicine Foundation Second‐Trimester Screening Group. An integrated model for the prediction of preeclampsia using maternal factors and uterine artery Doppler velocimetry in unselected low‐risk women. Am J Obstet Gynecol 2005; 193: 429–436.
- Papageorghiou AT, Yu CK, Erasmus IE, Cuckle HS, Nicolaides KH. Assessment of risk for the development of pre‐eclampsia by maternal characteristics and uterine artery Doppler. BJOG 2005; 112: 703– 709.
- International Institute for Population Sciences (IIPS) and ICF. 2017. National Family Health Survey (NFHS-4) 2015-16: India. Mumbai: IIPS
- Million Death Study Collaborators, Bassani DG, Kumar R, et al. Causes of neonatal and child mortality in India: a nationally representative mortality survey. Lancet. 2010; 376:1853–1860.
- Pre eclampsia. National Health Portal India. Accessed online from https://www.nhp.gov.in/disease/gynaecology-and-obstetrics/preeclampsia on March 1, 2020
- Antonisamy B, Sivaram M, Richard J, Rao PSS. Trends in Intra-uterine Growth of Single Live Births in Southern India. J Trop Pediatr. 1996. 339–341
- Pinheiro A, David A, Joseph B. Pregnancy weight gain and its correlation to birth weight. Indian J Med Sci. 2001;55:266–270.
- Maternal anthropometry and pregnancy outcomes. A WHO Collaborative Study: Introduction. Bull World Health Organ. 1995. S1–98.
- S. Murki, D. Sharma. Intrauterine Growth Retardation – A Review Article. J Neonatal Biol 2014, 3:3. DOI: 10.4172/2167-0897.1000135
- Deepak Sharma, Sweta Shastri, Nazanin Farahbakhsh & Pradeep Sharma (2016) Intrauterine growth restriction – part 1, The Journal of Maternal-Fetal & Neonatal Medicine, 29:24, 3977-3987.
- Saleem T, Sajjad N, Fatima S, Habib N, Ali SR, Qadir M. Intrauterine growth retardation–small events, big consequences. Ital J Pediatr. 2011 7;37:41. doi: 10.1186/1824-7288-37-41. PMID: 21899747; PMCID: PMC3177763.
- Chawanpaiboon S, Vogel JP, Moller AB, et al. Global, regional, and national estimates of levels of preterm birth in 2014: a systematic review and modelling analysis. Lancet Glob Health. 2019;7(1):e37–e46.
- Rolnik DL, Wright D, Poon LCY, et al. ASPRE trial: performance of screening for preterm pre-eclampsia [published correction appears in Ultrasound Obstet Gynecol. 2017 Dec;50(6):807]. Ultrasound Obstet Gynecol. 2017;50(4):492–495