Authors: Rijo M Choorakuttil, Devarajan P, Lalit K Sharma, Ramesh S Shenoy, Amel Antony, M.R. Balachandran Nair, Praveen K Nirmalan
- Rijo M Choorakuttil, National Coordinator for Samrakshan IRIA, AMMA Center for Diagnosis and Preventive Medicine, Kochi, Kerala, India
- Devarajan P, Nethra Scans and Genetic Clinic, Tiruppur, Tamil Nadu, India
- Lalit K Sharma, Raj Sonography & X- Ray Clinic, Baiju Choraha, Nayapura, Guna, Madhya Pradesh, India
- Ramesh S Shenoy, Consultant Radiologist, Lisie Hospital, Kochi, Ernakulam, India
- Amel Antony, Head, Department of Radiology, Lisie Hospital, Kochi, Ernakulam, India
- M.R. Balachandran Nair, Department of Radiology, Jubilee Mission Hospital, Thrissur, Kerala, India
- Praveen K Nirmalan, Chief Research Mentor, AMMA ERF, AMMA Center for Diagnosis and Preventive Medicine, Kochi, Kerala, India
Short Title: FetRADS-India
Corresponding Author: Rijo M Choorakuttil, National Coordinator for Samrakshan IRIA, AMMA Center for Diagnosis and Preventive Medicine, Kochi, Kerala, India. E mail: samrakshaniria@gmail.com
Key words: Fetal Radiology, Imaging, TIFFA, Pregnancy, Risk stratification
Abstract:
Context: Non-invasive radiology imaging techniques can be used to identify pregnant women at risk for adverse fetal and maternal events.
Aim: To describe the conceptual development of a comprehensive Fetal Radiology Assessment and Diagnostic Score- India (FetRADS-India) for risk stratification and early identification of pregnant women at risk for adverse fetal events.
Methods: The FetRADS-India item pool of variables pertinent to the score were generated using formative research including literature search to identify variables of interest to measure and content validation. A content validity ratio >0.49 was considered necessary for the retention of variables in the item pool. A composite score was generated combining the radiology imaging assessment and therapeutic weighted score.
Results: The FetRADS-India score has six levels with increasing scores indicating increasing risk for adverse fetal events. Subset scores predictive of obstetric adverse events based on assessment of fetal structure, growth and environment were developed.
Conclusions: The FetRADS-India categories have been developed, using content validation, for the risk stratification of pregnant women to help initiate appropriate early management for high risk cases including referral to advanced care or tertiary care units.
Introduction
National and regional health care delivery systems including the National Health Mission in India have a strong focus on improving maternal and child health.[1] These programs aim to improve the quality of obstetric and neonatal/child health care and surveillance with improved infrastructure, access, availability and affordability of services including antenatal and postpartum services and training of human resources to provide optimal care.[1] Improvements in health care delivery systems resulted in declining trends of maternal mortality, neonatal, infant and under-5 mortality and perinatal mortality rates in India although wide variations remain in maternal and child health by state, district and urban rural locations in India.[2-5] Maternal mortality in India has declined from 212 per 100,000 live births in 2007-09 to 178 per 100,000 live births in 2010-12.[1,2] Infant mortality rates in India have reduced from 47 per 1000 live births in 2010 to 42 per 1000 live births in 2012.[1,4,6,7] Neonatal mortality in India has reduced from 52 per 1000 live births in 1990 to about 28 per 1000 live births in 2013.[1,3,6,7] Under-5 mortality rates in India have also shown a declining trend, reducing from 55 per 1000 live births in 2011 to 29 per 1000 live births in 2015.[5-7] Despite these improvements, India remains a significant contributor towards the global maternal, neonatal, infant and under 5 mortality rates as well as the global stillbirth rates. Current perinatal mortality rates in India are estimated at 26 per 1000 live births (approximately 592,100 stillbirths) or 22.6% of the global still birth rates in 2015. [1,7]
The use of radiology, especially ultrasonography, is widely prevalent in obstetric care in India. Ultrasonography studies during pregnancy are used to identify fetal abnormalities that may impact the health of the fetus as well as that of the child post-delivery.[8-11] Appropriate radiology and imaging studies, including Doppler studies and placental studies, can be used to identify and predict adverse maternal conditions that may influence the well-being of the pregnant woman and the growing fetus. Radiological assessments and imaging thus supplement the obstetric decision making around childbirth. In this manuscript, we describe the development of a comprehensive Fetal Radiology Assessment and Diagnostic Score-India (FetRADS-India) focused on fetal structures, fetal growth and fetal environment that can be used for the early identification of pregnant women at risk for adverse maternal or fetal events in India and other developing economies.
Material and Methods
The FetRADS-India was developed using a multiphase approach. The first phase involved generation of the parameters of interest using formative research. A thorough literature search was done from January 1980 up to December 2015 using PubMed, MD Consult, Cochrane Library and EMBASE databases to identify articles of interest pertaining to radiological imaging for maternal and fetal well-being during pregnancy. The literature search was facilitated by the use of several key words or Medical Subject Headings (MeSH) terms. Several key words including but not limited to pregnancy, pregnancy risk factors, imaging in pregnancy, targeted imaging, fetal abnormalities, ultrasonography in pregnancy, 4D imaging studies, magnetic resonance imaging, placenta and placental abnormalities, Doppler studies, amniotic fluid, umbilical cord, cervix and uterine abnormalities, adnexal and ovarian masses, fetal growth, pregnancy induced hypertension, postpartum hemorrhage, maternal morbidity, maternal mortality, perinatal mortality, congenital abnormalities, and stillbirths were used to retrieve articles of interest. The literature obtained using these key words were critically evaluated to filter articles that could provide information on radiological and imaging parameters that potentially affect the health of a pregnant patient and to develop a list of radiological and imaging parameters that impact the health of the pregnant woman and the growing fetus. Parameters of interest included those that predicted fetal environment, fetal growth and fetal structure abnormalities and an increasing risk profile that ranged from low risk to a very high risk of adverse fetal events. The range of normality or abnormality for each parameter was also assessed through the literature search.
These parameters were further categorized based on the trimester of pregnancy or gestational age(s) at which the radiology study was performed. Identified radiology and imaging study parameters were further categorized based as structural and functional parameters influencing fetal structure, fetal growth and fetal environment. Structural parameters included structural integrity as well as location, while functional parameters explored the potential influence on specific functions (for example, flow velocities). Evidence pertaining to parameters was assessed in isolation, as well as in a sequential or simultaneous pattern. Thus, evidence was assessed for a single abnormal parameter, for multiple abnormal parameters in a single imaging study at a single time point, and for changing abnormalities in sequential imaging studies at multiple time points.
An item pool of parameters of interest, identified through the comprehensive search detailed above, was generated as part of the second phase of the development of FetRADS-India. These included structural and functional parameters at the three trimesters of pregnancy and involved sequential scans through the course of pregnancy. Briefly, the parameters of interest looked at the growth and structural integrity of the growing fetus in the first trimester (11-13th week), structural and functional integrity in the second trimester including Targeted Imaging for Fetal Anomaly (TIFFA) scan, and placental studies, fetal growth studies, Doppler studies and fetal well-being studies in the third trimester. Intrauterine growth restriction was defined as a fetal weight <10th centile for the gestational age and mean uterine and umbilical artery Doppler PI>95th centile. Early onset fetal growth restriction at 18-20 gestational weeks was defined as mean uterine and umbilical artery Doppler PI>95th centile and cerebroplacental ratio (CPR) <5th percentile with normal growth parameters. In the third trimester, late onset FGR was considered if one or more of the following three were present: fetal weight or AC is less than 10th percentile, mean uterine artery PI > 95th percentile or CPR < 5th percentile. In the third trimester, small for gestation age fetus was considered if the fetal weight was less than 10th percentile with normal Doppler parameters of uterine artery and CPR. A retroplacental hemorrhage, defined as a hypoechoeic area between the basal plate and myometrium that lifted the placental parenchyma towards the amniotic cavity, was categorized as small if the maximum diameter was less than 5 cm and large if the maximum diameter was more than 5 cm. The presence of septations, solid elements and colour Doppler low resistance waveform with PI < 1 and RI <0.4 are considered as some of the changes suggestive of malignancy. The thickness of the placenta is measured perpendicular to the basal and chorionic plates in the mid portion of the placenta. Anterior placentas >33mm and posterior placentas > 40mm were considered as thick. A single largest vertical pocket < 2 was considered as oligamnios, < 1 as severe and >8 as hydramnios at gestational ages < 28 weeks. For gestational ages > 28 weeks, a four quadrant amniotic fluid index of 5 to 8 was considered as mild, between 2 to 5 as moderate and less than 2 as severe reduction of amniotic fluid volume. The percentage of cervical funnelling and shape of the funnel (V or U shaped) and depth and width of the funnel was considered. Standard definitions were considered for structural anomalies; a complete list of structural anomalies and definitions is beyond the scope of this manuscript.
The third phase of the development of FetRADS-India involved assessing the content validity of the item pool of parameters generated as part of the second phase. Content validity is a non-statistical method that systematically examines the test content to determine if it covers a representative sample of the domain to be measured.[12-15] Content validity provides preliminary evidence on the construct validity of an instrument and is essential to establish reliability.[12-15] Each parameter was assessed as either not necessary, useful but not essential, or essential. A content validity ratio was ascertained for each parameter. Acceptance of the parameter was conditional on the content validity ration being greater than 0.49.[16]
Results
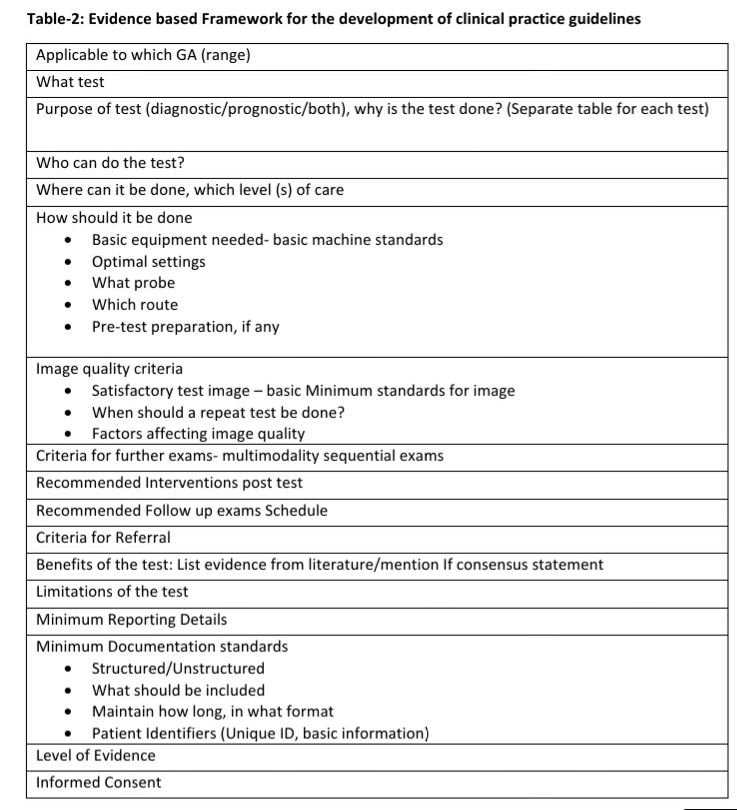
A six-stage composite scoring system based on the use of a radiological assessment score and a therapeutic weighted score was developed. The addition of a therapeutic weighted score ensured that higher scores reflected greater risk to maternal and/or fetal well-being and required more interventions or more intense surveillance. The lowest score indicated an incomplete or inadequate radiological study that limited the predictive ability (see Table-1). Several subset scores predictive of obstetric complications and obstetric monitoring were developed for each trimester (see Tables-2 to 4) to complete a comprehensive fetal assessment of structural anomalies, fetal growth and fetal environment.
Table-1: Broad scoring patterns of the Fetal Radiology Assessment and Diagnostic Score (FetRADS- India) system
| Score | Brief Description |
| FetRADS-0 | Incomplete or inadequate fetomaternal study due to conditions like
a) maternal body habitus (obesity/overweight) b) unfavourable fetal position c) oligamnios d) problems with equipment resolution and technical parameters e) informed consent not obtained |
| FetRADS-1 | Normal fetal growth and Maternal environment
No findings or predictors of obstetric risk or perinatal complications No structural anomalies or genetic markers visualized |
| FetRADS-2 | Findings and predictors of low risk obstetric and perinatal complications |
| FetRADS-3 | Presence of major genetic markers or multiple minor markers or a combination of both
Structural abnormalities that require antenatal interventions or definite postnatal interventions, with nil or minimal complications associated with interventions and good outcomes |
| FetRADS-4 | Findings of high risk obstetric and perinatal complications, high risk for genetic syndromes, structural abnormalities that has poor prognosis or severe complications |
| FetRADS-5 | Very high risk of obstetric and perinatal morbidity and mortality, fetal anomalies not sustainable with life or lethal. |
Table 2: FetRADS-India for 1st trimester (11-14 weeks scan)
| STRUCTURE | ENVIRONMENT | GROWTH | FetRADS-India |
| Previous h/o Downs/Aneuploidy | Obesity
Short stature | •Previous h/o Preeclampsia /Fetal growth restriction,
•Pre-existing Hypertension/Diabetes Mellitus | FetRADS 2 |
| NT >95th percentile for GA | Presence of adnexal Mass (es) | Increased combined Pulsatility Index of uterine artery and early diastolic notch | FetRADS 3 |
| •NT >3.5 mm,
•Cystic hygroma •Un ossified Nasal Bone •DV abnormality •TR present •Omphalocele, Gastrochisis | Presence of Mass (es) with red degeneration/torsion/ | FetRADS 4 | |
| Major Structural anomalies:
Anencephaly/ Neural tube defects/Holoproencephaly Monoventricle in heart Limb reduction defects | Adnexal masses with changes suggestive of malignancy | FetRADS 5 |
Table 3: FetRADS-India score for 2nd trimester scan
| STRUCTURE | ENVIRONMENT | GROWTH | FET RADS |
| •PrevIous h/o Downs/Aneuploidy
•Presence of Mild Urinary tract dilatation •Isolated Choroid Plexus cyst •Asymmetric lateral ventricles of brain, measurement > 8mm and < 10 mm •Single umbilical artery | • Oligo amnios (SDP <2 )
•Mild poly hydramnios (SDP: 8-12cm) •Increased Placental thickness •Low lying placenta-less than 2 cm from internal OS •Small retroplacental hemorrhage •Marginal Cord Insertion | •Previous h/o Preeclampsia/
FGR •Pre-existing Hypertension/ Diabetes Mellitus •EFW or AC < 10th percentile •Uterine Artery Mean PI >90th percentile | FetRADS 2 |
| •Mild Ventriculomegaly (>10mm) with no associated finding.
•Mild hypo or hypertelorism •Unilateral cleft lip and cleft palate •Unilateral or bilateral clubfoot with no associated anomalies. •Congenital pelvi-ureteric junction Obstruction •Unilateral multicystic dysplastic kidney with contralateral normal kidney and normal liquor •Arachnoid Cyst/Blake’s Pouch Cyst | • Oligo amnios (SDP <2)
•Moderate polyhydramnios (SDP: 12-15cm) •Circumvallate Placenta •Placenta previa-covering the OS •Large retroplacental hemorrhage > 2/3rd of surface •Short Cervix < 2.5 cm in transvaginal assessment •Presence of adnexal Mass (es) | •EFW or AC < 3rd percentile
•Uterine Artery •Mean PI >95th percentile •Cerebroplacental Ratio <5th percentile | FetRADS 3 |
| •Major genetic markers: Absent NB, Increased Nuchal fold thickness, ARSA
•Correctable congenital heart defects: VSD, ASD, TOF, TGA, CoA •Hydrops fetalis •Dandy walker malformation /vermian hypoplasia •Non-lethal skeletal dysplasias •Minor Neural tube defects | •Severe oligo hydramnios (SDP: <1cm);
•Severe Poly hydramnios; SDP>15cm•Funnelled Cervix with Bulging of Membranes, Anterior cervical angle > 105 degree •Velamentous cord insertion and Vasa Praevia, •Placental lucencies, Thinned out retroplacental clear space •Definite signs of placenta accreta spectrum •Presence of adnexal mass with red degeneration/ torsion/malignancy •Chorioangioma of placenta | •EFW or AC < 3rd percentile
•Uterine Artery Mean PI >99th percentile •Increased Umbilical artery PI: >95th percentile •Reversal of CPR | FetRADS 4 |
| •Neural tube defects associated with cerebellar or brainstem defects,
•Cerebellar dysplasia •Agenesis of corpus callosum •HLHS, AVSD, Ebsteins anomaly •Lethal skeletal dysplasias •Bilateral multi cystic renal dysplasia, •Post urethral valve •Tracheo-oesophageal fistula/ atresia | •Anhydramnios (Nil liquor)
•Partial hydatidiform mole | •Ductus Venosus Doppler abnormality | FetRADS 5 |
Table 4- FetRADS-India score for 3rd trimester*
| EFW Percentiles | Uterine Artery PI | CPR & Umbilical Artery Doppler | AOI | DV | FetRADS |
| 10-95 | Normal | Normal | Normal | Normal | FetRADS-1 |
| <10 or >95 | Normal | Normal | Normal | Normal | FetRADS-2 |
| <10 | >95th percentile | CPR <5th percentile | Normal | Normal | FetRADS-3 |
| <3 | Normal | Normal | Normal | Normal | |
| 10-95 | >95th percentile | CPR <5th percentile | Normal | Normal | |
| <3 /<10 | >95th percentile | CPR <5th percentile
Umbilical Artery absent or reversal of EDV | Abnormal | Normal | FetRADS-4 |
| <3 /<10 | >95th percentile | CPR <5th percentile
Umbilical Artery absent or reversal of EDV | Abnormal | Abnormal or Biophysical score (BPP) <4 | FetRADS-5 |
* Structural anomaly score and environment score are similar for the 2nd and 3rd trimester. Evolving structural abnormalities are also considered based on the 2nd trimester structural anomaly scores. For gestational ages > 28 weeks, a four quadrant amniotic fluid index of 5 to 8 was considered as mild, between 2 to 5 as moderate and less than 2 as severe reduction of amniotic fluid volume. Both oligoamnios and polyhydramnios in the 3rd trimester are considered similar to 2nd trimester categories.
Discussion
Radiology image reporting and data systems have been used to improve diagnostic and prognostic assessments in various conditions including thyroid disorders, breast disorders, and prostate disorders and for liver disorders. [17-21] Risk stratification systems have been used for several clinical conditions to provide a framework for clinical decision making and resource allocation. The use of scoring systems may help improve consistency in evidence-based management across practices and institutions.
The focus of perinatal health care delivery in India has been on improving physical infrastructure, human resource training and access, availability and affordability of services. Evidence for the use of diagnostic tests, including biochemical tests and genetic markers, primarily to determine maternal and fetal morbidity in the course of pregnancy is increasing. [22-25] However, biochemical and genetic tests have certain limitations. Availability, affordability and accessibility to such expert services is currently a limitation in developing economies. Additionally, the invasive nature of sample collection and turnaround time to receiving the report are limitations for biochemical and genetic tests especially in developing economies where follow up visits are suboptimal.
Non-invasive radiology and imaging studies are currently part of antenatal care for the pregnant woman and are usually done in each trimester of pregnancy. Targeted Imaging for Fetal Anomalies scan (TIFFA or fetal anomaly scan) done in pregnancy provides useful information on the structural integrity of the growing fetus including fetal anatomy and presence of any fetal anomalies.[26-29] Congenital anomalies are identified as one of the top ten causes for mortality in children and a TIFFA Scan can provide early information on the presence of lethal and non-lethal congenital anomalies.[30] Information from the TIFFA scan, when combined with information from the first trimester ultrasound study and the third trimester ultrasound study, provides a comprehensive assessment of the fetal environment and growth that aids obstetricians in clinical decision making about childbirth. Integrating Doppler studies in the first trimester to detect pregnancy induced hypertension and to differentiate and manage fetal growth restriction in the third trimester and the use of supplemental multimodality imaging and synergistic fetal imaging/radiology with Ultrasound, placental exams, 3D or 4D imaging studies and Magnetic Resonance Imaging completes a comprehensive assessment of the fetus. Sequential scans (at least one in each trimester) are advised for optimal results, however, not all pregnant women may opt for or undergo sequential scans.
Technical aspects including types of equipment, resolutions, and technical competency maybe potential limitations in the use of the FetRADS-India categories. However, these potential limitations maybe reduced as several training programs and workshops are planned as part of a new initiative (Samrakshan) by the Indian Radiological & Imaging Association to improve competency in fetal ultrasonography and multimodality imaging. [31-33] An ultrasound study may not always be possible in a pregnant woman due to maternal and fetal factors like maternal obesity, unfavourable fetal positions, and oligamnios. We have considered this potential limitation and assigned a FIRADS 0 score for incomplete or inadequate studies. Women who are assigned a FIRADS-0 score should be referred to a higher center for further evaluation with higher end machines and multimodality approaches including fetal MRI at appropriate intervals.
In this manuscript, we have described the development of a comprehensive scoring system for the early identification and risk stratification of the pregnant woman, with particular relevance to the Indian subcontinent context, which can be used to aid decision making around childbirth without the need for additional resources. The scoring system can be incorporated into routine antenatal care and provide the obstetrician with information for the early identification of at-risk pregnancies potentially reducing perinatal mortality. The scoring system aims to provide a systematic sequential assessment framework for risk moving through several multi modality imaging techniques. The scoring system also expands the focus of fetal assessment to fetal environment and growth beyond the current focus on identifying abnormalities. The details of the validity, reliability, diagnostic effectiveness and discriminant ability of the FetRADS-India categories, tested in a multi center clinical setting, will be presented in a separate manuscript. Individual parameters and scores assigned to each parameter, based on statistical analyses, will be described to provide composite and specific scores to predict adverse events. The final FetRADS-India categories and parameters in each category will be determined on completion of the multicentre study.
In conclusion, FetRADS-India has been developed through a systematic process including content validation to provide a systematic pathway for the risk stratification of pregnant women and to initiate appropriate early management for high risk cases including referral to advanced care or tertiary care units. The scoring system is dynamic and can be supplemented through the use of appropriate multimodality imaging techniques.
References
- Negandhi PH, Neogi BS, Chopra S, Phogat A, Sahota R, Gupta R, et al. Improving reporting of infant deaths, maternal deaths and stillbirths in Haryana, India. Bull World Health Organ 2016;94:370-375
- NITI Aayog [Internet]. New Delhi. National Institute for Transforming India, Government of India. [cited April 1, 2018]. Available from http://niti.gov.in/content/maternal-mortality-ratio-mmr-100000-live-births#
- NITI Aayog [Internet]. New Delhi. National Institute for Transforming India, Government of India. [cited April 1, 2018]. Available from http://niti.gov.in/content/neo-natal-mortality-rate-nmr-1000-live-births
- NITI Aayog [Internet]. New Delhi. National Institute for Transforming India, Government of India. [cited April 1, 2018]. Available from http://niti.gov.in/content/infant-mortality-rate-imr-1000-live-births
- NITI Aayog [Internet]. New Delhi. National Institute for Transforming India, Government of India. [cited April 1, 2018]. Available from http://niti.gov.in/content/under-5-mortality-rate-u-5mr-1000-live-births
- Ram U, Jha P, Ram F, Kumar K, Awasthi S, Shet A et al. Neonatal, 1-59 month, and under- 5 mortality in 597 Indian districts, 2001 to 2012: estimates from national demographic and mortality surveys. Lancet Glob Health 2013;1:e219-26
- Sankar MJ, Neogi BS, Sharma J, Chauhan M, Srivatsava R, Prabhakar PK et al. Status of Newborn Health in India. J Perinatol 2016;36:S3-S8
- Bhide A, Acharya G, Bilardo CM, Brezinka C, Cafici D, Hernandez-Andrade E et al. ISUOG practice guidelines: use of Doppler ultrasonography in obstetrics. Ultrasound Obstet Gynecol 2013;41: 233-39
- Benson CB, Doubilet PM. The history of imaging in obstetrics. Radiology 2014;273: S92-110
- Shah S, Adedipe A, Ruffatto B, Backlund BH, Sajed D, Rood K et al. BE-SAFE: Bedside sonography for assessment of the fetus in emergencies: educational intervention for late-pregnancy obstetric ultrasound. West J Emerg Med 2014;15:636-40
- ISUOG Education Committee recommendations for basic training in obstetric and gynecological ultrasound. Ultrasound Obstet Gynecol 2014;43: 113-6
- Rubio DM, Berg-Weger M, Tebb SS, Lee ES, Rauch S. Objectifying Content Validity: Conducting a content validity study in social work research. Social Work Research 2003;27:94-104
- Newman I, Lim J, Pineda F. Content Validity using a mixed methods approach: Its application and development through the use of a table of specifications methodology. Journal of Mixed Methods Research 2013;7:243-60
- Wynd CA, Schmidt B, Schaefer MA. Two quantitative approaches for estimating content validity. West J Nurs Res 2003;25:508-18
- Yaghmale F. Content Validity and its estimation. Journal of Medical Education 2003;3:25-7
- Lawshe CH. A quantitative approach to content validity. Personnel Psychology 1975;28:563-75
- Tessler FN, Middleton WD, Grant EG, Hoang JK, Berland LL, Teefey SA et al. ACR Thyroid Imaging, Reporting and Data Systems (TI-RADS): White Paper of the ACR TI-RADS committee. J Am Coll Radiol 2017;14:587-95
- Mercado CL. BI-RADS update. Radiol Clin North Am 2014;52:481-7
- Purysko AS, Rosenkratz AB, Barentsz JO, Weinreb JC, Macura KJ. PI-RADS Version 2: A pictorial update. Radiographics 2016;36:1354-72
- Rosenkratz AB, Oto A, Turkbey B, Westphalen AC. Prostate Imaging and reporting System (PI-RADS), Version2: A Critical Look. AJR AM J Roentgenol 2016;206:1179-83
- Mitchell DG, Bruix J, Sherman M, Sirlin CB. LI-RADS (Liver Imaging Reporting and Data System): summary, discussion and consensus of the LI-RADS Management Working Group and future directions. Hepatology 2015; 61:1056-65
- Heazell AE, Whitworth M, Duley L, Thornton JG. Use of biochemical tests of placental function for improving pregnancy outcome. Cochrane Database Syst Rev 2015;11:CD011202
- Cicero S, Bindra R, Rembouskos G, Spencer K, Nicolaides KH. Integrated ultrasound and biochemical screening for trisomy 21 using fetal nuchal translucency, absent fetal nasal bone, free beta-hCG, and PAPP-A at 11 to 14 weeks. Prenat Diagn 2003;23:306-10
- Cicero S, Spencer K, Avgidou K, Faiola S, Nicolaides KH. Maternal serum biochemistry at 11-13 (+6) weeks in relation to the presence or absence of the fetal nasal bone on ultrasonography in chromosomally abnormal fetuses: an updated analysis of integrated ultrasound and biochemical screening. Prenat Diagn 2005;25:977-83
- Cecatti JG, Souza RT, Sulek K, Costa ML, Kenny LC, McCowan LM et al. Use of metabolomics for the identification and validation of clinical biomarkers for preterm birth: Preterm SAMBA. BMC Pregnancy Childbirth 2016;16:212
- Gupta S, Timor-Tritsch IE, Oh C, Chervenak J, Monteagudo A. Early second trimester sonography to improve the fetal anatomic survey in obese patients. J Ultrasound Med 2014;33:1579-83
- Sonek J, Croom C. Second trimester ultrasound markers of fetal aneuploidy. Clin Obstet Gynecol 2014;57:159-81
- VanDorsten JP, Hulsey TC, Newman RB, Menard MK. Fetal anomaly detection by second trimester ultrasonography in a tertiary center. Am J Obstet Gynecol 1998;178:742-9
- Pooh RK, Kujrak A. 3D/4D sonography moved prenatal diagnosis of fetal anomalies from the second to the first trimester of pregnancy. J Matern Fetal Neonatal Med 2012;25:433-55
- Global Burden of Disease Pediatrics Collaboration, Kyu HH, Pinho C, Wagner JA, Brown JC, Bertozzi-Villa A et al. Global and National Burden of Diseases and Injuries among Children and Adolescents between 1990 and 2013: Findings from the Global Burden of Disease 2013 Study. JAMA Pediatr 2016;170:267-87
- Choorakuttil RM, Devarajan P, Bavaharan R, Jain N, Sharma LK, Nagar S. Samrakshan: Rationale for universal 1st trimester screening to identify pregnant women at risk for preterm preeclampsia. Accessed online from Journal of Fetal Radiology at http://fetalradiology.in/2019/11/14/2908/ on Dec 2, 2019
- Shenoy S, Choorakuttil RM, Bavaharan R, Devarajan P, Nirmalan PK. Mobile Learning as an Integral Part of Samrakshan IRIA national program. Accessed online from Journal of Fetal Radiology at http://fetalradiology.in/2019/11/25/mobile-learning-as-an-integral-part-of-samrakshan-iria-national-program/ on Dec 2, 2019
- Choorakuttil RM, Patel H, Bavaharan R, Devarajan P, Kanhirat S, Shenoy RS, Tiwari OP, Sodani RK, Sharma LK, Nirmalan PK. Samrakshan: An Indian radiological and imaging association program to reduce perinatal mortality in India. Indian J Radiol Imaging 2019;29:412-7