Authors: Anjali Gupta, Renu Sharma, Lalit K Sharma, Akanksha Baghel, Bavaharan Rajalingam, Shilpa R Satarkar, Devarajan Palanisamy, Neelam Jain, Rijo M Choorakuttil & Praveen K Nirmalan for Team Samrakshan
Author Affiliations:
- Anjali Gupta, Anjali Ultrasound and Colour Doppler centre, 2nd floor, Shanti Madhuban Plaza, Delhi Gate, Agra, Uttar Pradesh, India,
- Renu Sharma, Dr Renu’s Diagnostic Center,E 6 Basant Vihar, Sikar, Rajasthan, India
- Lalit K Sharma, Raj Sonography & X- Ray Clinic, Baiju Choraha, Nayapura, Guna, Madhya Pradesh, India
- Akanksha Baghel, Baghel Sonography Center. Front of Janpat Office, near District Hospital, Harda, Madhya Pradesh, India
- Bavaharan R, Fetocare Magnum Imaging and Diagnostics, Trichy, Tamil Nadu, India
- Shilpa R Satarkar, Antarang Sonography and Colour Doppler Center, Satarkar Hospital, Plot 20. Tilaknagar, Aurangabad, Maharashtra, India
- Devarajan P, Nethra Scans and Genetic Clinic, Tiruppur, Tamil Nadu, India
- Neelam Jain, Jain Ultrasound centre, C-112, B Block, Dispensary road Sonari, Jamshedpur Jharkhand, India
- Rijo M Choorakuttil, National Coordinator for Samrakshan IRIA, AMMA Center for Diagnosis and Preventive Medicine, Kochi, Kerala, India
- Praveen K Nirmalan, Chief Research Mentor, AMMA ERF, AMMA Center for Diagnosis and Preventive Medicine, Kochi, Kerala, India
Short Title: Colour Doppler Ultrasound in 3rd Trimester
Corresponding Author: Rijo M Choorakuttil, National Coordinator for Samrakshan IRIA, AMMA Center for Diagnosis and Preventive Medicine, Kochi, Kerala, India. E-mail: rijomc@gmail.com
Keywords: Uterine artery PI, Umbilical artery PI, Middle Cerebral Artery PI, Cerebro-Placental Ratio, 3rd trimester, Fetal Growth Restriction
Abstract
Aim: To determine the magnitude of abnormal Doppler studies and the impact of integrating colour Doppler studies in the third trimester of pregnancy on the diagnosis of fetal growth restriction.
Methods: Pregnant women were screened with colour Doppler studies in addition to routine trimester specific ultrasound exams in the third trimester as part of the Samrakshan program. The presence of one or more of the following- Mean Uterine Artery PI >95th percentile, umbilical artery PI >95th percentile, middle cerebral artery PI <5th percentile and Cerebro-Placental ratio <5th percentile was considered abnormal. Fetal Growth Restriction (FGR) was categorized and management recommendations proposed based on the Barcelona staging method.
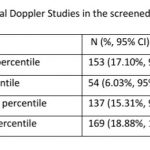
Results: The data records of 895 consecutive pregnant women screened in the 3rd trimester from 15 districts of 7 states in India from July 2019 to January 2020 were analysed. An abnormal Doppler study was identified in 273 (30.50%, 95% CI: 27.58, 33.60) of the 895 screened women. The use of EFW <10th percentile identified 198 (95% CI: 19.53, 24.96) fetuses and abnormal liquor identified 153 (95% CI: 14.77, 19.70) fetuses as potentially at risk. The proportion of fetuses identified with FGR showed a statistically significant (p<0.001) reduction from 22.12% (based on EFW <10th percentile) to 14.97% with the integration of colour Doppler studies..
Conclusion: The integration of colour Doppler studies in 3rd trimester screening protocol led to a significant increase in the number of fetuses identified as potentially “at risk” for fetal compromise and a significant reclassification of FGR fetuses.
Introduction
The National Family Health Survey (NFHS)-4 reported a perinatal mortality rate of 36 per 1000 pregnancies in India.[1] The NFHS-4 reported a neonatal mortality rate of 30 per 1000 live births and a still birth rate of 0.7. [1] Prematurity and low birth weight were identified as the major causes for neonatal mortality in India. [2]
Early antenatal detection and appropriate management of fetal growth restriction is important to minimize perinatal mortality. Samrakshan hypothesizes that a systematic, protocol-based approach for the earlier identification and management of preterm pre-eclampsia (PE) and fetal growth restriction (FGR) can reduce perinatal mortality in India. [3] To achieve this, Samrakshan uses trimester specific protocols integrating colour Doppler studies with routine trimester specific ultrasound exams. [3] We have previously described the first trimester protocol followed by Samrakshan.[4] In the third trimester, Samrakshan integrates colour Doppler studies and utilizes a Doppler Ultrasound based stage-based protocol for the early identification and management of fetal growth restriction (FGR) in singletons pregnancies.[3] In this manuscript, we report preliminary results and compare the incidence of fetuses identified as “at risk” for potential compromise in the screened population based on Colour Doppler Ultrasound and the conventionally used combination of estimated fetal weight centiles and amniotic fluid volume .
Methods
The processes in the third trimester protocol of Samrakshan have been described in a previous publication. [5] Briefly, every woman screened in the Samrakshan program is assigned a unique identification number at the first visit. The same number was used for further documentation through the course of pregnancy. In the third trimester, details of parity and conception, and presence of co-morbidity were documented.
Routine ultrasound exams were done to estimate fetal weight (EFW) and fetal biometric parameters and plotted on growth charts. The amniotic fluid index (AFI) and/or the single deepest vertical pocket was used to assess adequacy of liquor. A four-quadrant amniotic fluid index of 5 to 8 cm was considered as mild, between 2 to 5 cm as moderate and less than 2 cm as severe reduction of amniotic fluid volume. A single deepest pocket <2 cm was considered as oligamnios and >8 cm as polyhydramnios.
Colour Doppler studies of the right and left uterine artery and umbilical artery was performed for each woman. The protocols used for colour Doppler studies have been described in a previous publication.[5] Uterine artery Doppler indices were assessed in the 3rd trimester using a transabdominal approach [6] and a mean Uterine Artery PI >95th centile was considered as abnormal. The pulsed‐wave Doppler sampling gate was set to be narrow (set at approximately 2 mm) and right and left uterine arteries were identified at the apparent crossover with the external iliac arteries. After the arteries were identified, pulsed‐wave Doppler was used to obtain the waveforms. Umbilical Artery Doppler indices were assessed at a free loop cord and Umbilical Artery PI >95th centiles were considered abnormal. [7] Fetal middle cerebral artery (MCA) Doppler waveforms were measured to obtain peak systolic volume (PSV) using auto trace or manual calipers [7] and a MCA PI <5th centile was considered as abnormal. The pulsed wave Doppler gate was placed at the proximal third of MCA close to its origin in the internal carotid artery keeping the angle between direction of blood flow and ultrasound beam as close to 0 as possible. The Cerebro-Placental Ratio (CPR) was estimated by dividing the MCA Doppler indices by the umbilical artery Doppler indices. A CPR PI <5th was considered as abnormal for the Samrakshan program. [3,5] Although the S/D ratio, RI, and PI have been reported when computing the CPR, the PI is the computation of choice. [8-15]. Ductus Venosus (DV) assessments and absent or reversed end diastolic velocity were assessed if mean uterine artery PI and/or CPR were abnormal.[5]
Fetal Growth was staged using a composite model involving fetal weight and Doppler indices based on the model proposed by Figeuras, at al.[16] A fetus was considered as small for gestational age (SGA) if the EFW was 3-10th percentile with normal Doppler indices.
Data in the Samrakshan program was entered online using trimester specific forms and stored in a password protected electronic database. Data analysis was performed using MS Excel and the Open Source Epidemiologic Statistics for Public Health program. Categorical variables are presented as frequency and proportions and continuous variables as the mean (SD). 95% confidence intervals (CI) are presented around point estimates. Bivariate analysis was used to explore associations with abnormal Doppler studies. Associations with abnormal Doppler studies were further explored by including factors found significant in the bivariate analysis in a multivariate regression analysis model and expressed as an adjusted odds ratio. AP value <0.05 was considered as statistically significant.
Results
We analysed the data records of 895 consecutive pregnant women screened in the 3rd trimester from 15 districts of 7 states in India as part of the Samrakshan program from July 2019 to January 2020. The mean age (SD) of subjects was 27.19 (4.86) years (range 18 to 47 years). The mean gestational age (SD) at examination was 33.96 (3.10) weeks.
An abnormal Doppler study was identified in 273 (30.50%, 95% CI: 27.58, 33.60) of the 895 screened women. One hundred and ninety eight women (22.12%, 95% CI: 19.53, 24.96) had a fetus with an estimated fetal weight <10th percentile and 153 (17.09%, 95% CI: 14.77, 19.70) women had abnormal liquor. Abnormal Doppler studies were significantly associated (see Table 1) with nulliparity (p=0.001), development of PE in the 3rd trimester (P <0.001), estimated fetal weight <10th percentile (p<0.001) and abnormal liquor (p<0.001).
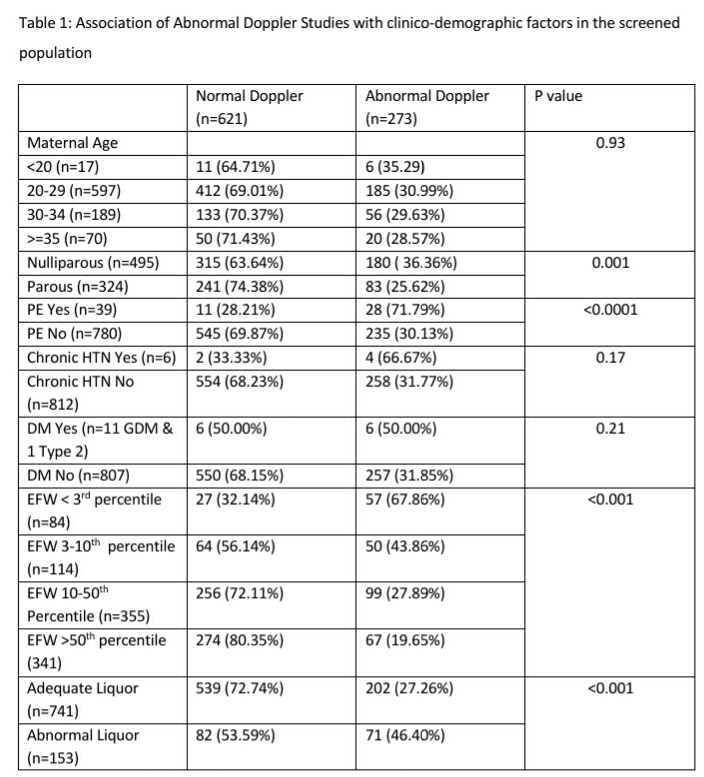
The association of nulliparity with abnormal Doppler studies persisted in a multivariate logistic regression analysis model that included factors found significant in bivariate analysis ( adjusted Odds Ratio 1.71, 95% CI: 1.42, 2.07, p <0.001).
Sixty four (32.32%, 95% CI: 26.2, 39.12) fetuses with EFW <10th percentile (n=198) were identified as “SGA without FGR” (EFW 3-10th percentile and Normal Doppler study) in the screened population. A “true” FGR, considered as EFW <3rd percentile irrespective of Doppler status and abnormal Doppler study in fetuses with EFW 3-10th percentile, was present in 134 (67.68%, 95% CI: 60.88, 73.80) of the 198 women with EFW <10th percentile or 14.97% (95% CI: 12.78, 17.46) of the screened population.
Ninety nine (27.89%, 95% CI: 23.48, 32.77) fetuses with EFW 10-50th percentile and 67 (19.65%, 95% CI: 15.78, 24.19) fetuses with EFW >50th percentile had an abnormal Doppler study.
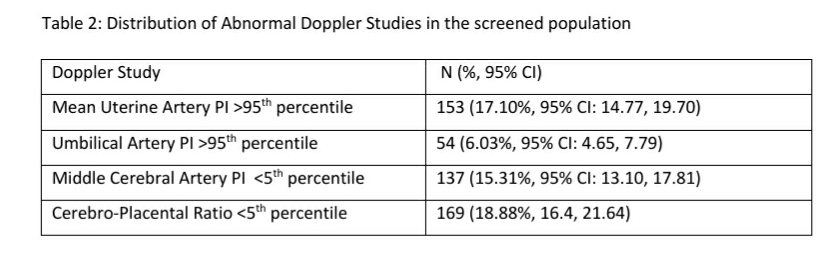
Abnormal CPR and mean uterine artery PI were the most common abnormal Doppler study (see Table 2) in the screened population (n=895)
Details of childbirth of these 895 screened women are being collected as an ongoing process and is available for 192 (21.45%) childbirths till end of January 2020. There were 4 neonatal deaths in this series. All the 4 neonatal deaths were in Stage 3 FGR fetuses and had been advised cardiotocographic monitoring, steroids for lung maturation and urgent delivery of the child and had preterm births. 3 of these 4 neonatal deaths occurred in fetuses with EFW <3rd percentile and one neonatal death occurred in a fetus with EFW 10-50th centile with late and rapid deterioration of Doppler indices and fetal health at 35 gestational weeks. There was one stillbirth in this series in a stage 3 FGR with EFW <3rd centile, maternal PE in a nulliparous woman whom was advised immediate delivery at 31 gestational weeks for maternal and fetal indications but had a family related delay ( recommendation of immediate childbirth to childbirth interval >24 hours) of childbirth and delivered a stillborn fetus while being transferred to the operation theatre for a caesarean section.
Details of childbirth are currently available only for 9 (14.06%) of the 64 fetuses with normal Doppler and EFW 3-10th percentile (SGA). There was no perinatal mortality in this group. Two of these 9 fetuses were delivered at 32 to 33 gestational weeks due to deteriorating maternal health (pre-eclampsia). After excluding these two cases, the mean gestational age at childbirth was 36.43 gestation weeks and the mean birth weight was 2471.43 grams.
Discussion
An important outcome of the integration of colour Doppler studies in the third trimester screening protocol is the ability to differentiate FGR from SGA fetuses in the group with EFW <10th percentile. In this series, colour Doppler studies helped to differentiate 64 fetuses (32.32%, 95% CI: 26.2, 39.12) of the 198 fetuses with EFW <10th percentile as SGA and were advised follow up screening as per the regular schedule in the 3rd trimester. The proportion of fetuses identified with FGR showed a statistically significant (p<0.001) reduction from 22.12% (based on EFW <10th percentile) to 14.97% with the integration of colour Doppler studies. An additional benefit from the integration of colour Doppler studies is the ability to stage FGR based on severity and to recommend appropriate stage based management. The preliminary results from Samrakshan till date show a neonatal mortality rate of 20.94 per 1000 live births and a perinatal mortality rate of 26.04 per 1000 pregnancies compared to 30 and 36 respectively reported in the NFHS-4.
The integration of colour Doppler studies in 3rd trimester screening protocol led to a significant (p<0.001) increase in the number of fetuses identified as potentially “at risk” for fetal compromise. The use of colour Doppler identified 273 fetuses as potentially at risk compared to 198 fetuses with an EFW <10th percentile and 153 pregnancies with an abnormal liquor status. The integration of Doppler studies identified abnormal Doppler indices in 99 (27.89%, 95% CI: 23.48, 32.77) fetuses with EFW 10-50th percentile and 67 (19.65%, 95% CI: 15.78, 24.19) fetuses with EFW >50th percentile. Approximately one-third of fetuses with EFW 10-50th percentile and nearly one-fifth of fetuses with EFW >50th percentile had an abnormal Doppler study.
The integration of colour Doppler studies thus significantly reduced the proportion of fetuses diagnosed as FGR but led to a significant increase in the pool of potentially “at risk” fetuses that need closer surveillance. These results raise two important questions. One, is it necessary to offer closer surveillance for this subgroup of fetuses with EFW >10th centile and abnormal Doppler studies (that led to the significant increase in the number of fetuses needing surveillance) ? Two, how do we follow up this subgroup?
Both preeclampsia and FGR show an impaired quality and quantity of maternal vascular response to placentation. Several studies have reported an increased impedance to flow in the uterine arteries and consequent adverse pregnancy and perinatal outcomes in women with pregnancy induced hypertension. [17-20] Umbilical artery Doppler studies has been used since several decades in the identification of FGR. However, umbilical artery Doppler studies identify primarily severe placental disease and fails to pick up mild placental disease which constitute a proportion of early-onset cases and virtually all instances of late-onset FGR. [21] It has been reported, based on pathology studies, that increased impedance in the umbilical arteries is seen only when at least 60% of the placental vascular bed is obliterated [22] This may explain the relatively low incidence of abnormal umbilical artery Doppler study in this series as 87.67% of the identified FGR were classified as Stage 1 FGR.
There is an increase in the blood supply to the brain, myocardium and the adrenal glands and reduction in the perfusion of the kidneys, gastrointestinal tract and the lower extremities in fetal hypoxia. The partial pressures of oxygen and carbon dioxide may play a role through their action on chemoreceptors allowing preferential delivery of nutrients and oxygen to vital organs, and compensating for diminished placental resources. Several studies have reported a curvilinear relationship between impedance in cerebral vessels and the state of fetal oxygenation. The progressive fall in impedance reached its lowest point 2 weeks before the onset of late fetal heart rate decelerations suggesting that the maximum degree of vascular adaptation to hypoxemia occurs earlier than the critical degree of impairment of fetal oxygenation. [23, 24] The middle cerebral artery provides information about brain vasodilation and is used as a surrogate for fetal hypoxia. Abnormal MCA is a late manifestation and is particularly valuable for the identification and prediction [25,26] of adverse outcome among late-onset FGR independent of the umbilical artery Doppler status. The CPR is a diagnostic index that combines information from the umbilical artery and the MCA and is the best test to pick up the effects of fetal adaptation.[16] A proportion of cases with abnormal CPR may not progress to baseline hypoxia and remain only with abnormal CPR. However, once baseline hypoxia is established, the placental reserves are minimal and progression to fetal deterioration may occur quickly. This results in a high risk of severe deterioration or intrauterine fetal death after 37 weeks due to a combination of a higher susceptibility to hypoxia of the term-mature fetus and the more common presence of contractions at term. [16] Abnormal CPR was the most common abnormal Doppler study in our series.
It may be pragmatic to initiate a close surveillance of the fetus considering the association of abnormal Doppler studies with adverse perinatal outcomes. As part of the Samrakshan protocol, we decided to follow up fetuses with EFW 10-50th percentile and abnormal Doppler studies applying the stage-based management proposed by Figeuras et al [16] for FGR to this group. Further study of the effects of abnormal Doppler studies in this subgroup of fetuses with EFW 10-50th percentile and childbirth management strategies are needed.
Abnormal Doppler Studies are possible even in LGA fetuses especially in the presence of diabetes mellitus. Studies have reported that CPR is useful also in AGA and LGA babies and that changes in umbilical Doppler according to the fetal size suggest the need to adjust the Doppler reference ranges according to the fetal weight percentile.[27,28] Nearly one fifth of fetuses at the higher percentiles of EFW (>50th percentile) had an abnormal Doppler study.
The Samrakshan protocol is opening up several interesting directions for fetal radiology research into clinical management strategies that can reduce perinatal mortality in India. Determining follow up assessment schedules and child birth strategies in fetuses with EFW >10th percentile and abnormal Doppler studies, especially fetuses with EFW 10-50th percentile is a priority given the large number of fetuses in that group. The diagnostic effectiveness of abnormal Doppler studies linked to adverse perinatal outcomes in this population in diverse settings is also a priority.
References
- International Institute for Population Sciences (IIPS) and ICF. 2017. National Family Health Survey (NFHS-4) 2015-16: India. Mumbai: IIPS
- Million Death Study Collaborators, Bassani DG, Kumar R, et al. Causes of neonatal and child mortality in India: a nationally representative mortality survey. Lancet. 2010; 376:1853–1860.
- Choorakuttil RM, Patel H, Bavaharan R, Devarajan P, Kanhirat S, Shenoy RS, Tiwari OP, Sodani RK, Sharma LK, Nirmalan PK. Samrakshan: An Indian radiological and imaging association program to reduce perinatal mortality in India. Indian J Radiol Imaging 2019;29:412-7
- Choorakuttil RM, Devarajan P, Rajalingam B, Jain N, Sharma LK, Nagar S, et al. Samrakshan: Rationale for universal 1st trimester screening to identify pregnant women at risk for preterm preeclampsia. Accessed online from http://fetalradiology.in/2019/11/14/2908/ on February 1, 2020
- Bavaharan R, Choorakuttil RM, Ahuja B, Gupta A, Sharma LK, Baghel A, et al. Routine 3rd Trimester Colour Doppler Ultrasound in Fetuses with Estimated Fetal Weight 10-50th centiles in India- Preliminary Results from the Samrakshan Program. Accessed online from http://fetalradiology.in/2020/01/16/routine-3rd-trimester-colour-doppler-ultrasound-in-fetuses-with-estimated-fetal-weight-10-50th-centiles-in-india-preliminary-results-from-the-samrakshan-program/ on February 1,2020
- Sotiriadis A, Hernandez‐Andrade E, da Silva Costa F, et al. ISUOG Practice Guidelines: Role of ultrasound in screening for and follow‐up of pre‐eclampsia. Ultrasound Obstet Gynecol. 2019; 53: 7– 22.
- Bhide A, Acharya G, Bilardo CM, et al. ISUOG practice guidelines: use of Doppler ultrasonography in obstetrics. Ultrasound Obstet Gynecol. 2013;41(2):233–239
- Arbeille, P., Body, G., Saliba, E. et al. Fetal cerebral circulation assessment by Doppler ultrasound in normal and pathological pregnancies. Eur J Obstet Gynecol Reprod Biol. 1988; 29: 261-273
- Gramellini, D., Folli, M.C., Raboni, S., Vadora, E., and Merialdi, A. Cerebral-umbilical Doppler ratio as a predictor of adverse perinatal outcome. Obstet Gynecol. 1992; 79: 416-420
- Arias, F. Accuracy of the middle-cerebral-to-umbilical-artery resistance index ratio in the prediction of neonatal outcome in patients at high risk for fetal and neonatal complications. Am J Obstet Gynecol. 1994; 171: 1541-1545
- Bahado-Singh, R.O., Kovanci, E., Jeffres, A. et al. The Doppler cerebroplacental ratio and perinatal outcome in intrauterine growth restriction. Am J Obstet Gynecol. 1999; 180: 750-756
- Baschat, A.A. and Gembruch, U. The cerebroplacental Doppler ratio revisited. Ultrasound Obstet Gynecol. 2003; 21: 124-127
- Odibo, A.O., Riddick, C., Pare, E., Stamilio, D.M., and Macones, G.A. Cerebroplacental Doppler ratio and adverse perinatal outcomes in intrauterine growth restriction: evaluating the impact of using gestational age-specific reference values. J Ultrasound Med. 2005; 24: 1223-12
- Ebbing, C., Rasmussen, S., and Kiserud, T. Middle cerebral artery blood flow velocities and pulsatility index and the cerebroplacental pulsatility ratio: longitudinal reference ranges and terms for serial measurements. Ultrasound Obstet Gynecol. 2007; 30: 287-296
- Morales-Rosello, J., Khalil, A., Morlando, M., Papageorghiou, A., Bhide, A., and Thilaganathan, B. Changes in fetal Doppler indices as a marker of failure to reach growth potential at term. Ultrasound Obstet Gynecol. 2014; 43: 303-310
- Figueras F, Gratacós E. Update on the diagnosis and classification of fetal growth restriction and proposal of a stage-based management protocol. Fetal Diagn Ther. 2014;36(2):86–98.
- Campbell S, Griffin DR, Pearce JM, Diaz-Recasens J, Cohen-Overbeek T,Wilson K, Teague MJ. New Doppler technique for assessing uteroplacental blood flow. Lancet 1983;26:675–7
- Trudinger BJ, Giles WB, Cook CM. Uteroplacental blood flow velocity-time waveforms in normal and complicated pregnancy. Br J Obstet Gynaecol 1985;92:39–45
- Campbell S, Pearce JM, Hackett G, Cohen-Overbeek T, Hernandez C. Qualitative assessment of uteroplacental blood flow: an early screening test for high risk pregnancies. Obstet Gynecol 1986;68: 649–53
- Fleisher A, Schulman H, Farmakides G, Bracero L, Rochelson B, Koenigsberg M. Uterine artery Doppler velocimetry in pregnant women with hypertension. Am J Obstet Gynecol 1986;154:806–13
- Oros D, et al: Longitudinal changes in uterine, umbilical and fetal cerebral Doppler indices in late-onset small-for-gestational age fetuses. Ultrasound Obstet Gynecol 2011;37:191-195.
- Giles WB, Trudinger BJ, Baird PJ. Fetal umbilical artery flow velocity waveforms and placental resistance: pathological correlation. Br J Obstet Gynaecol 1985;92:31–8
- Arduini D, Rizzo G, Romanini C. Changes of pulsatility index from fetal vessels preceding the onset of late decelerations in growth-retarded fetuses. Obstet Gynecol 1992;79:605–10
- Potts P, Connors G, Gillis S, Hunse C, Richardson B. The effect of carbon dioxide on Doppler flow velocity waveforms in the human fetus. J Dev Physiol 1992;17:119–23
- Eixarch E, et al: Neurodevelopmental outcome in 2-year-old infants who were small-for-gestational age term fetuses with cerebral blood flow redistribution. Ultrasound Obstet Gynecol 2008;32:894-899.
- Hershkovitz R, et al: Fetal cerebral blood flow redistribution in late gestation: identification of compromise in small fetuses with normal umbilical artery Doppler. Ultrasound Obstet Gynecol 2000;15:209-212.
- Sirico A, Diemert A, Glosemeyer P, Hecher K. Prediction of adverse perinatal outcome by cerebroplacental ratio adjusted for estimated fetal weight. Ultrasound Obstet Gynecol. 2018;51(3):381–386. doi:10.1002/uog.17458
- Sirico A, Rizzo G, Maruotti GM, et al. Does fetal macrosomia affect umbilical artery Doppler velocity waveforms in pregnancies complicated by gestational diabetes?. J Matern Fetal Neonatal Med. 2016;29(20):3266–3270. doi:10.3109/14767058.2015.1121479