Authors: Rajalingam Bavaharan, Rijo M Choorakuttil, Bhupendra Ahuja, Anjali Gupta, Lalit K Sharma, Akanksha Baghel, Shweta Nagar, Devarajan Palanisamy, Ramesh S Shenoy, Praveen K Nirmalan
Author Affiliations
1. Bavaharan R, Fetocare Magnum Imaging and Diagnostics, Trichy, Tamil Nadu, India
2. Rijo M Choorakuttil, National Coordinator for Samrakshan IRIA, AMMA Center for Diagnosis and Preventive Medicine, Kochi, Kerala, India
3. Bhupendra Ahuja, Dr. Ahuja Ultrasonography and Colour Doppler Center, Delhi Gate, Agra, (Dr. Sarkar Market), Uttar Pradesh, India
4. Anjali Gupta, Anjali Ultrasound and Colour Doppler centre, 2nd floor, Shanti Madhuban Plaza, Delhi Gate, Agra, Uttar Pradesh, India,
5. Lalit K Sharma, Raj Sonography & X- Ray Clinic, Baiju Choraha, Nayapura, Guna, Madhya Pradesh, India
6. Akanksha Baghel, Baghel Sonography Center. Front of Janpat Office, near District Hospital, Harda, Madhya Pradesh, India
7. Shweta Nagar, Dr. Shweta Nagar’s Ultrasound Clinic & Imaging Centre, Flat no. 101, Princes Center, above Mama Loca cafe, 6/3 New Palasia, Indore, Madhya Pradesh, India
8. Devarajan P, Nethra Scans and Genetic Clinic, Tiruppur, Tamil Nadu, India
9. Ramesh S Shenoy, Consultant Radiologist, Lisie Hospital, Ernakulam, Kerala, India.
10. Praveen K Nirmalan, Chief Research Mentor, AMMA ERF, AMMA Center for Diagnosis and Preventive Medicine, Kochi, Kerala, India
Short Title: 3rd trimester colour Doppler
Corresponding Author: Rijo M Choorakuttil, National Coordinator for Samrakshan IRIA, AMMA Center for Diagnosis and Preventive Medicine, Kochi, Kerala, India. E mail: samrakshaniria@gmail.com
Keywords: Uterine artery PI, Umbilical artery PI, Middle Cerebral Artery PI, Cerebro-Placental Ratio, 3rd trimester
Abstract
Aim: To determine the incidence and distribution of abnormal Doppler studies in fetuses with estimated fetal weight 10-50th centiles in the 3rd trimester
Methods: Doppler Ultrasound studies were done as part of the routine 3rd trimester specific ultrasound exams in the Samrakshan Program. Pregnant women underwent colour Doppler ultrasound assessments of the uterine artery, umbilical artery, middle cerebral artery (MCA) and Cerebro-Placental ratio (CPR) was computed. Ductus Venosus (DV) assessments were done if uterine artery or CPR was abnormal. Mean Uterine Artery PI and Umbilical Artery PI >95th centiles and MCA and CPR <5th centiles were considered abnormal.
Results: Six hundred and seventy five pregnant women were screened in the third trimester of pregnancy from July 2019 to December 2019 as part of the Samrakshan program in 5 states of India. Two hundred and fifty one (37.18%, 95% CI: 33.62, 40.89) of these 675 women had a fetus with EFW between the 10th and 50th centile and abnormal Doppler studies were recorded in 76 (30.28%, 95% CI: 24.93, 36.22) of these 251 fetuses.
Conclusion: Routine use of 3rd trimester Doppler Ultrasound studies resulted in the identification of an additional 30.28% fetuses in the EFW 10-50th centile or 11.26% of overall screened population (n=76 of 675 fetuses) to be at risk for adverse perinatal outcomes. These AGA fetuses were in a predominantly low risk population and would not have been identified for closer surveillance in the absence of Doppler studies
Introduction
Samrakshan is a national program of the Indian Radiological & Imaging Association (IRIA) that aims to reduce perinatal mortality in India.[1] Pre-eclampsia (PE) and Fetal Growth Restriction (FGR) are the conditions of primary interest for Samrakshan. The National Family Health Survey-4 (NFHS-4), a nationally representative survey carried out in 2015-16 in India, reported a perinatal mortality rate of 36 per 1000 pregnancies. [2] NFHS-4 reported a still birth rate of 0.7% and a neonatal mortality rate of 30 per 1000 live births in India, and a birth weight <2500 grams in 18.2% of live births.[2] A major proportion (41.77%) of neonatal deaths in India was attributable to prematurity and low birth weight.[3]
The antenatal detection of fetal growth restriction and fetuses at risk for adverse perinatal outcomes is important to minimize perinatal mortality . Birth weight is a predictor for perinatal mortality and shows an inverted J pattern with a long and steep mortality slope for babies with birth weight <10th centile and a short slope for birth weights >90th centile.[4-11] This led to the design of approaches targeting perinatal mortality that are focused on the extremes of birth weight (the <10th centile and >90th centile). There is increasing evidence that the 50-90th birth weight centile might be optimum for term births. [12] Birth weight centiles <50th centile had significantly higher perinatal deaths even among fetuses classified as appropriate for gestational age (AGA). [12] Babies with a 10-25th birthweight centile had a two-fold increased risk of perinatal death and babies with a 25-50th birthweight centile had 1.5 times higher risk for perinatal mortality. [12]
The evidence of higher perinatal mortality even among babies with birthweight centiles <50, or a subset of AGA babies, has tremendous implications for India that has a large number of annual births. Even a small shift in the perinatal mortality rates in this category can lead to a large change in terms of absolute numbers of deaths averted. Samrakshan utilizes a Doppler Ultrasound based stage based protocol for the management of Fetal Growth Restriction. [1, 13] The routine use of Colour Doppler Ultrasound studies was extended to all 3rd trimester singletons pregnancies screened as part of the Samrakshan program to derive initial evidence on the distribution of abnormal Doppler studies in fetuses with an estimated fetal weight (EFW) >10th centile as well. This manuscript presents the results of colour Doppler studies in the 3rd trimester of pregnancy among fetuses with an EFW between the 10-50th centiles.
Materials and Methods
Samrakshan utilizes an opportunistic screening program aimed at identifying pregnancies at risk for adverse perinatal outcomes among pregnant women undergoing radiology and imaging services in the antenatal period. [14] Trimester specific screening protocols were utilized and each pregnant woman in the program was assigned a unique identification number.[14]
In the third trimester, clinico-demographic details including parity, prior history of PE, development of PE in the current pregnancy, gestational age at diagnosis of PE, and co-morbidity in the current pregnancy was collected from each pregnant woman. Routine ultrasound exams were done to estimate fetal weight (EFW) and fetal biometric parameters. Colour Doppler studies of the right and left uterine artery and umbilical artery was performed for each woman. Uterine artery Doppler indices were assessed in the 3rd trimester using a transabdominal approach. Uterine arteries were identified using color flow mapping and gentle sideways tilt of the transducer from the midline infra-umbilical region of the maternal abdomen. The pulsed‐wave Doppler sampling gate was set to be narrow (set at approximately 2 mm) and right and left uterine arteries were identified at the apparent crossover with the external iliac arteries. After the arteries were identified, pulsed‐wave Doppler was used to obtain the waveforms. When at least three similar consecutive waveforms were obtained, the Pulsatility Index (PI) was measured. A peak systolic velocity >60 cm/s was used to verify that the uterine artery was being examined.[15] A mean Uterine Artery PI >95th centile was considered as abnormal. Umbilical Artery Doppler indices were assessed at a free loop cord and Umbilical Artery PI >95th centiles were considered abnormal.[16]
Fetal middle cerebral artery (MCA) Doppler waveforms were measured after initially obtaining and magnifying an axial section of the brain that included the thalamus and the sphenoid bone wings. Colour flow mapping was then used to identify the circle of Willis and the proximal MCA. The pulsed wave Doppler gate was placed at the proximal third of MCA close to its origin in the internal carotid artery keeping the angle between direction of blood flow and ultrasound beam as close to 0 as possible. At least 3 but fewer than 20 wave forms were recorded with the highest point of the waveform considered as the peak systolic volume (PSV). The PSV was measured using auto trace or manual calipers.[16] A MCA PI <5th centile was considered as abnormal.
The Cerebro-Placental Ratio (CPR) was estimated by dividing the MCA Doppler indices by the umbilical artery Doppler indices. A CPR PI <5th was considered as abnormal for the Samrakshan program. Although the S/D ratio, RI, and PI have been reported when computing the CPR, more recently the PI is the computation of choice. [17-24]
The amniotic fluid index (AFI) and/or the single deepest vertical pocket was used to assess adequacy of liquor. Fetal Growth was staged using a composite model involving fetal weight and Doppler indices based on the model proposed by Figeuras, at al.[13] Ductus Venosus (DV) assessments and absent or reversed end diastolic velocity were assessed if mean uterine artery PI and/or CPR were abnormal. Fetal breathing and fetal heart rate was documented and an assessment of decelerating fetal health was made based on these indices.
Data in the Samrakshan program is entered online using trimester specific forms and stored in a password protected electronic database. Data analysis was performed using MS Excel and the Open Source Epidemiologic Statistics for Public Health program. Categorical variables are presented as frequency and proportions and continuous variables as the mean (SD). 95% confidence intervals (CI) are presented around point estimates.
Results
Six hundred and seventy five pregnant women were screened in the third trimester of pregnancy from July 2019 to December 2019 as part of the Samrakshan program in 5 states of India. Two hundred and fifty one (37.18%, 95% CI: 33.62, 40.89) of these 675 women had a fetus with EFW between the 10th and 50th centile, 285 (42.22%, 95% CI: 38.55, 45.98) women had a fetus with EFW >50th centile and 139 (20.59%, 95% CI: 17.71, 23.81) women had a fetus with EFW <10th centile.
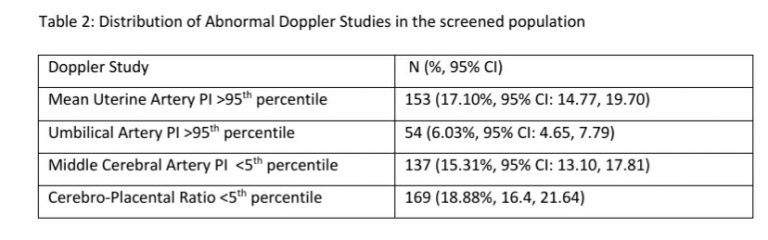
Abnormal Doppler studies were recorded in 81 (58.27%, 95% CI: 49.96, 66.14) fetuses with EFW <10th centile, in 76 (30.28%, 95% CI: 24.93, 36.22) fetuses with EFW 10-50th centile and in 56 (19.65%, 95% CI: 15.45, 24.65) fetuses with EFW >50th centile. Further analysis is presented for the subgroup of fetuses (n=251) with EFW 10-50th centiles.
Table 1: Clinico-demographic details of the 251 pregnant women with EFW 10-50th centiles
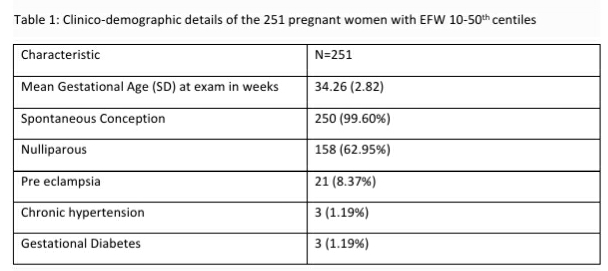
The population of pregnant women with fetuses having EFW 10-50th centiles in the third trimester was primarily a low risk population with a clinically low risk for adverse perinatal outcomes (see Table 1). Inadequate liquor volume was determined in 30 (11.95%, 95% CI: 8.50, 16.55) of the 251 fetuses with EFW 10-50th centiles, however, abnormal Doppler studies were found in significantly (p<0.001) higher proportion (n=76, 30.28%) of these 251 “low risk” pregnant women (See Table-2). Only one fetus each showed abnormal fetal heart rate and abnormal fetal breathing and three fetuses were determined to have decelerating fetal health as they transitioned between stages of FGR.
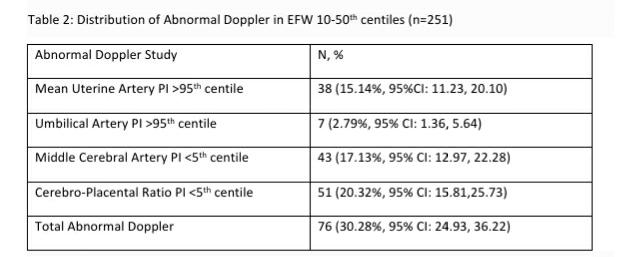
Table 2: Distribution of Abnormal Doppler in EFW 10-50th centiles (n=251)
Discussion
Small fetuses have a higher risk of adverse perinatal outcomes. Conventionally, small fetuses have been defined based on an EFW <10th centile. Small fetuses can be further categorized as fetal growth restriction (a failure of the fetus to reach its growth potential) or as constitutionally small for gestational age (SGA). [13] Growth restricted fetuses are usually associated with Doppler signs that reflect hemodynamic redistribution as a fetal adaptation response to under nutrition/hypoxia, histological and biochemical signs of placental disease and a higher risk of preeclampsia.[13] The management of FGR in the third trimester of pregnancy focuses on monitoring the fetal condition so that childbirth and induction of delivery can be optimally timed. FGR fetuses can be delivered electively when lung maturation can be presumed, or earlier if signs of fetal deterioration are observed. [13] SGA fetuses, on the other hand, do not have an abnormal Doppler reflective of an abnormal fetal environment and usually have perinatal outcomes similar to that of normally grown fetuses, and can be delivered at or closer to term. [13]
The antenatal identification of fetuses at increased risk for hypoxia related events remains suboptimal despite the widespread use of intrapartum or admission cardiotocography and amniotic fluid assessments. [25-28] Adverse events related to fetal hypoxia, such as cerebral palsy and stillbirth, are more prevalent in FGR secondary to placental insufficiency but is not uncommon in AGA babies. [29,30] Even AGA infants can be affected by placental insufficiency and fail to meet their genetic growth potential at term. [13,24] Studies have also shown placental histological abnormalities typical of FGR in about 25% of term AGA pregnancies, suggesting the presence of occult chronic placental insufficiency. [13,24] Fetal Doppler assessment can be of value in detecting pregnancies that are AGA, yet complicated by placental insufficiency, although much of the focus has been on abnormal Doppler studies in SGA or FGR babies.
CPR reflects the interaction of altered blood flow to the brain and is manifested by increased diastolic flow as the result of cerebrovascular dilation resulting from hypoxia and increased placental resistance, resulting in decreased diastolic flow of the umbilical artery. [17-24,31]When these alterations occur, the increased diastolic flow of the MCA is manifest by a decrease in the systolic/diastolic ratio (S/D), resistance index (RI), and the pulsatility index (PI), whereas these measurements are increased in the umbilical artery as the result of increased resistance to blood flow as the result of placental pathology.[17-24,31] An abnormal CPR has been reported, by Prior et al, in 11% of AGA fetuses, and the incidence of abnormal CPR was significantly higher among those who underwent cesarean delivery for fetal distress and was a better predictor for an emergency cesarean delivery than an abnormal UA or MCA, independent of fetal size.[32] Morales-Rosello et al, reported that the UA and venous pH were significantly lower in AGA newborns who had an abnormal CPR than AGA fetuses with a normal CPR suggesting that CPR can be used to assess the risk of intrapartum fetal distress requiring cesarean delivery, or acidemia at birth, in AGA fetuses. [33]
Khalil A, et al, [34] reported that the risks of operative delivery for fetal compromise and neonatal intensive care unit admission are significantly increased in both SGA and AGA fetuses when CPR was low at term. The rate of operative delivery for presumed fetal compromise was even higher in AGA fetuses with low CPR compared to SGA fetuses with normal CPR, indicating that CPR is more strongly associated with fetal compromise due to placental insufficiency than is birth weight.
The antenatal diagnosis of FGR using fetal biometry alone is no longer a clinically viable option and the use of fetal Doppler assessment is a potentially better marker. A normally sized (AGA) fetus at term exposed to hypoxemia from placental insufficiency invariably has a short latency to delivery, and therefore does not really have enough time to manifest SGA as a feature. In contrast, Doppler-detectable cerebral redistribution, a fundamental physiological response to hypoxemia in preterm FGR and reflective of placental insufficiency, can be detected at term. An abnormal CPR is also a better predictor of adverse outcome than the biophysical profile as CPR can detect adverse changes much earlier than the biophysical profiles.[13,24,35,36]
The possibility of AGA fetuses with occult or undetected placental insufficiency has implications on the current UA and MCA Doppler normal ranges at term. These reference ranges can be influenced by the inclusion of AGA pregnancies suffering from undetected placental insufficiency and hence may not be appropriate. Khalil A, et al, [34] used the concept of optimal CPR established from fetuses on the upper BW centiles and showed a stronger relationship between umbilical cord pH and CPR compared to BW. These findings challenge the convention of using EFW to assess the at-risk fetus at term and suggest that fetal arterial Doppler assessment may have a more important role.
In this study, we found that one third of singleton pregnancies with EFW 10-50th centile had an abnormal Doppler study. A CPR <5th centile was the most common abnormal Doppler finding (20.32%, 95% CI: 15.81,25.73) in this cohort. The majority of the 251 pregnancies with EFW 10-50th centile were clinically low risk and were candidates to be recommended routine antenatal care in the 3rd trimester. Twenty one women with pregnancy induced hypertension and three each with chronic hypertension and GDM may have been considered for closer surveillance. An additional 11.95% pregnancies would have been considered for closer surveillance based on inadequate liquor volume. The use of routine Doppler Ultrasound studies, not restricted to umbilical artery PI alone but inclusive of mean uterine artery PI, MCA and importantly CPR, increased the identification of at-risk fetuses to 30.28% in this low risk cohort.
The preliminary results from Samrakshan are consistent with evidence from the global literature and show the potential for AGA babies to have hitherto undetected placental insufficiency that cannot be identified by fetal size estimates or liquor volume. We have not correlated the identification of at-risk fetuses with perinatal outcomes; that process is ongoing and will be presented as a separate manuscript. However, fetuses identified as at risk based on Doppler studies have been followed up based on the staging method proposed by Figeuras, et al [13] for FGR. Further studies are required to determine childbirth strategies in this cohort with EFW 10-50th centiles and abnormal Doppler and to determine how much they may differ from fetuses with EFW <10th centile with abnormal Doppler studies.
In conclusion, the routine use of 3rd trimester Doppler Ultrasound studies resulted in the identification of an additional 30.28% fetuses in the EFW 10-50th centile or 11.26% of overall screened population (n=76 of 675 fetuses) to be at risk for adverse perinatal outcomes; these pregnancies are being closely followed up. Considering that these fetuses had EFW in the AGA range and were in a predominantly low risk population, they would not have been picked up for closer surveillance in the absence of Doppler studies.
References
1. Choorakuttil RM, Patel H, Bavaharan R, Devarajan P, Kanhirat S, Shenoy RS, Tiwari OP, Sodani RK, Sharma LK, Nirmalan PK. Samrakshan: An Indian radiological and imaging association program to reduce perinatal mortality in India. Indian J Radiol Imaging 2019;29:412-7
2. International Institute for Population Sciences (IIPS) and ICF. 2017. National Family Health Survey (NFHS-4) 2015-16: India. Mumbai: IIPS
3. Million Death Study Collaborators, Bassani DG, Kumar R, et al. Causes of neonatal and child mortality in India: a nationally representative mortality survey. Lancet. 2010; 376:1853–1860.
4. Haig D. Mediations on birth weight: is it better to reduce the variance or increase the mean? Epidemiology. 2003;14(4): 490–492.
5. Zhivotovsky LA, Feldman MW. On the difference between mean and optimum of quantitative characters under selection. Evolution. 1992;46(5): 1574–1578.
6. Karn MN, Penrose LS. Birth weight and gestation time in relation to maternal age, parity and infant survival. Ann Eugen. 1951;16: 147–164.
7. Wilcox AJ, Russell IT. Birthweight and perinatal mortality: II. On weight-specific mortality. Int J Epidemiol. 1983;12(3): 319–325.
8. Wilcox AJ. On the importance–and the unimportance–of birthweight. Int J Epidemiol. 2001;30: 1233–1241.
9. Basso O, Wilcox AJ, Weinberg CR. Birth weight and mortality: causality or confounding? Am J Epidemiol. 2006;164: 303–311.
10. Hernández-Díaz S, Wilcox AJ, Schisterman EF, Hernán M. From causal diagrams to birth weight-specific curves of infant mortality. Eur J Epidemiol. 2008;23: 163–166.
11. Schisterman EF, Hernández-Díaz S. Invited commentary: simple models for a complicated reality. Am J Epidemiol. 2006;164: 312–314
12. Francis JH, Permezel M, Davey MA. Perinatal mortality by birthweight centile. Aust N Z J Obstet Gynaecol. 2014;54(4):354–359.
13. Figueras F, Gratacós E. Update on the diagnosis and classification of fetal growth restriction and proposal of a stage-based management protocol. Fetal Diagn Ther. 2014;36(2):86–98.
14. Choorakuttil RM, Devarajan P, Rajalingam B, Jain N, Sharma LK, Nagar S, et al. Samrakshan: Rationale for universal 1st trimester screening to identify pregnant women at risk for preterm preeclampsia. Accessed online from http://fetalradiology.in/2019/11/14/2908/ on December 24, 2019
15. Sotiriadis A, Hernandez‐Andrade E, da Silva Costa F, et al. ISUOG Practice Guidelines: Role of ultrasound in screening for and follow‐up of pre‐eclampsia. Ultrasound Obstet Gynecol. 2019; 53: 7– 22.
16. Bhide A, Acharya G, Bilardo CM, et al. ISUOG practice guidelines: use of Doppler ultrasonography in obstetrics. Ultrasound Obstet Gynecol. 2013;41(2):233–239
17. Arbeille, P., Body, G., Saliba, E. et al. Fetal cerebral circulation assessment by Doppler ultrasound in normal and pathological pregnancies. Eur J Obstet Gynecol Reprod Biol. 1988; 29: 261-273
18. Gramellini, D., Folli, M.C., Raboni, S., Vadora, E., and Merialdi, A. Cerebral-umbilical Doppler ratio as a predictor of adverse perinatal outcome. Obstet Gynecol. 1992; 79: 416-420
19. Arias, F. Accuracy of the middle-cerebral-to-umbilical-artery resistance index ratio in the prediction of neonatal outcome in patients at high risk for fetal and neonatal complications. Am J Obstet Gynecol. 1994; 171: 1541-1545
20. Bahado-Singh, R.O., Kovanci, E., Jeffres, A. et al. The Doppler cerebroplacental ratio and perinatal outcome in intrauterine growth restriction. Am J Obstet Gynecol. 1999; 180: 750-756
21. Baschat, A.A. and Gembruch, U. The cerebroplacental Doppler ratio revisited. Ultrasound Obstet Gynecol. 2003; 21: 124-127
22. Odibo, A.O., Riddick, C., Pare, E., Stamilio, D.M., and Macones, G.A. Cerebroplacental Doppler ratio and adverse perinatal outcomes in intrauterine growth restriction: evaluating the impact of using gestational age-specific reference values. J Ultrasound Med. 2005; 24: 1223-12
23. Ebbing, C., Rasmussen, S., and Kiserud, T. Middle cerebral artery blood flow velocities and pulsatility index and the cerebroplacental pulsatility ratio: longitudinal reference ranges and terms for serial measurements. Ultrasound Obstet Gynecol. 2007; 30: 287-296
24. Morales-Rosello, J., Khalil, A., Morlando, M., Papageorghiou, A., Bhide, A., and Thilaganathan, B. Changes in fetal Doppler indices as a marker of failure to reach growth potential at term. Ultrasound Obstet Gynecol. 2014; 43: 303-310
25. Clark, S.L. and Hankins, G.D.V. Temporal and demographic trends in cerebral palsy–fact and fiction. Am J Obstet Gynecol. 2003; 188: 628-633
26. Alfirevic, Z., Devane, D., and Gyte, G.M. Continuous cardiotocography (CTG) as a form of electronic fetal monitoring (EFM) for fetal assessment during labor. Cochrane Database Syst Rev. 2006; 3: CD006066
27. Devane, D., Lalor, J.G., Daly, S., McGuire, W., and Smith, V. Cardiotocography versus intermittent auscultation of fetal heart on admission to labor ward for assessment of fetal well-being. Cochrane Database Syst Rev. 2012; 2: CD005122
28. Kushtagi, P. and Deepika, K.S. Amniotic fluid index at admission in labor as predictor of intrapartum fetal status. J Obstet Gynaecol. 2011; 31: 393-395
29. Arcangeli, T., Thilaganathan, B., Hooper, R., Khan, K.S., and Bhide, A. Neurodevelopmental delay in small babies at term: a systematic review. Ultrasound Obstet Gynecol. 2012; 40: 267-275
30. Malin, G., Morris, R., Riley, R., Teune, M., and Khan, K. When is birthweight at term abnormally low? A systematic review and meta-analysis of the association and predictive ability of current birthweight standards for neonatal outcomes. BJOG. 2014; 121: 515-526
31. Baschat, A.A., Gembruch, U., and Harman, C.R. The sequence of changes in Doppler and biophysical parameters as severe fetal growth restriction worsens. Ultrasound Obstet Gynecol. 2001; 18: 571-577
32. Prior, T., Mullins, E., Bennett, P., and Kumar, S. Prediction of intrapartum fetal compromise using the cerebroumbilical ratio: a prospective observational study. Am J Obstet Gynecol. 2013; 208: 124.e1-124.e6
33. Morales-Rosello, J., Khalil, A., Morlando, M., Bhide, A., Papageorghiou, A., and Thilaganathan, B. Poor neonatal acid-base status in term fetuses with low cerebroplacental ratio. Ultrasound Obstet Gynecol. 2015; 45: 156-161
34. Khalil AA, Morales-Rosello J, Morlando M, et al. Is fetal cerebroplacental ratio an independent predictor of intrapartum fetal compromise and neonatal unit admission?. Am J Obstet Gynecol. 2015;213(1):54.e1–54.e10.
35. Sebire, N.J. Detection of fetal growth restriction at autopsy in non-anomalous stillborn infants. Ultrasound Obstet Gynecol. 2014; 43: 241-244
36. Unterscheider, J., Daly, S., Geary, M.P. et al. Optimizing the definition of intrauterine growth restriction: the multicenter prospective PORTO study. Am J Obstet Gynecol. 2013; 208: 290.e1-290.e6