Authors: Devarajan P, Lalit K Sharma, Shilpa Satarkar, Anjali Gupta, Suresh Saboo, M.V. Kameswar Rao, B.M. Swain, Premraj Satapathy, Akanksha Baghel, Avni KP Skandhan, Rijo M Choorakuttil, Praveen K Nirmalan for the Samrakshan Team
Author Affiliations:
- Devarajan P, Nethra Scans and Genetic Clinic, Tiruppur, Tamil Nadu, India
- Lalit K Sharma, Raj Sonography & X- Ray Clinic, Baiju Choraha, Nayapura, Guna, Madhya Pradesh, India
- Shilpa R Satarkar, Antarang Sonography and Colour Doppler Center, Satarkar Hospital, Plot 20, Tilaknagar, Aurangabad, Maharashtra, India
- Anjali Gupta, Anjali Ultrasound and Colour Doppler centre, 2nd floor, Shanti Madhuban Plaza, Delhi Gate, Agra, Uttar Pradesh, India
- Suresh Saboo, Professor and HOD, Department of Radiology, JIJU, IIMS Medical College, Jalna, Maharashtra, India
- M.V.Kameswar Rao, Professor, Department of Radiodiagnosis, MKCG Medical College, Berhampur, Odisha, India
- B.M.Swain, Associate Professor, Department of Radiodiagnosis, SCB Medical College, Cuttack, Odisha, India
- Premraj Satapathy, Balaji Diagnostic and Research Center, Courtpeta Square, Behrampur, Ganjam, Odisha, India
- Akanksha Baghel, Baghel Sonography Center. Front of Janpat Office, near District Hospital, Harda, Madhya Pradesh, India
- Avni KP Skandhan, Aster MIMS, Kottakkal, Kerala, India
- Rijo M Choorakuttil, National Coordinator for Samrakshan IRIA, AMMA Center for Diagnosis and Preventive Medicine, Kochi, Kerala, India
- Praveen K Nirmalan, Chief Research Mentor, AMMA ERF, AMMA Center for Diagnosis and Preventive Medicine, Kochi, Kerala, India
Abstract
Aim: To describe the baseline results of a 1st trimester screening protocol using a competing risk Bayesian model-based algorithm in the Samrakshan program
Methods: Each woman screened in the Samrakshan program is assigned a unique identification number to identify longitudinal records. Clinico-Demographic details, mean arterial blood pressure and Mean Uterine Artery PI were measured for women screened in Samrakshan. These details were input into an online calculator of the Fetal Medicine Foundation to estimate risk for preterm preeclampsia and fetal growth restriction for each woman. A cut-off of 1 in 150 was used to categorize risk and high risk women were recommended 150 mg of aspirin at bedtime.
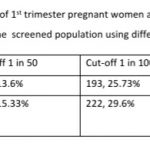
Results: 168 (22.4%, 95% CI: 19.56, 25.52) women were at high risk for both preterm PE and FGR; an additional 96 (12.8%, 95% CI: 10.6, 15.38) women at high risk only for preterm PE and 95 (12.67%, 95% CI: 10.48, 15.24) women at high-risk only for FGR. 122 women (16.27%, 95% CI: 13.80, 19.08) had a gestation age specific mean uterine artery PI >95th percentile based on the global reference standard. Mean arterial blood pressure >95th percentile and mean uterine artery PI >95th percentile was significantly associated with a higher risk for preterm PE. The proportion of women that are considered as high risk shows a significant reduction from 35.2% to 25.73% if we use a cut-off of 1 in 100 for preterm PE and from 45.3% to 29.6% for FGR. These proportions fall significantly further when we use a risk of 1 in 50, to 13.6% for preterm PE and 15.33% for FGR.
Conclusion: The 1st trimester screening protocol of Samrakshan identified a large proportion of women at high risk for preterm PE and FGR. Further studies are needed to determine longitudinal progression, regional variations in mean arterial pressure and mean uterine artery PI and the diagnostic effectiveness of different risk estimation cut-offs.
Keywords: Samrakshan, India, Pregnancy, Screening, First trimester, Preeclampsia, Fetal Growth Restriction
Introduction
Pre-eclampsia (PE) and fetal growth restriction (FGR) are major contributors to the high perinatal mortality rate in India.[1-3] It is possible to identify women at risk for preterm PE and FGR through a competing risk Bayesian model-based algorithm in the 1st trimester of pregnancy. [4] Low dose aspirin, at a dose of 150mg daily, once at bedtime has been proven to minimize or delay the onset of preterm PE among high risk women. [5,6] The effectiveness of low dose aspirin is optimal if it is started between 11 -14 weeks of pregnancy. [4-6]
Samrakshan Is a program of Indian Radiological and Imaging Association (IRIA) that aims to reduce perinatal mortality in India. [7] Samrakshan utilizes a specific protocol for the early identification, in the 1st trimester of pregnancy, of women at risk for preterm PE and FGR.[8] Women identified as high risk are recommended low dose aspirin, 150 mg once daily at bedtime.[8] We have earlier reported on the baseline results of the 2nd and 3rd trimester screening of Samrakshan and child birth outcomes. [9-11] In this manuscript, we report on the baseline results of the 1st trimester screening using the Samrakshan protocol.
Methods
We have previously described the rationale and processes of the 1st trimester screening protocol of Samrakshan. [8] Briefly, each pregnant woman screened in Samrakshan is assigned a unique identification number that is used for longitudinal follow up. Clinico-demographic details of the pregnant woman was collected and included age, height and weight of the woman, and past and current obstetric history. Systolic and diastolic blood pressure were measured twice in each arm using a digital blood pressure measuring apparatus to derive a mean arterial pressure (MAP) for each woman. [12] A colour Doppler study of the uterine artery was done [4], in addition to the routine biometry measurements and NT scans, for each woman. The gestational age specific mean Uterine artery Pulsatility Index (PI) was determined for each woman.
The clinico-demographic details, the MAP and Mean Uterine Artery Doppler PI were entered into the Fetal Medicine Foundation (FMF) online calculator available online at https://fetalmedicine.org/research/assess/preeclampsia/first-trimester. A personalized risk estimate of preterm PE and FGR was determined for each woman. It was decided, based on consensus, to use a 1 in 150 cut-off to identify women at risk in the initial phases of Samrakshan. Pregnant women identified as “at risk” were recommended Low dose Aspirin, 150 mg at bedtime. [8] The risk estimates and recommendation was shared with the obstetrician who was the primary treating physician.
Data entered into the online FMF calculator and the personalized risk estimate were transcribed into an offline data form that was stored with the medical records of the woman. The offline form was subsequently entered into a patient de-identified online database for further analysis using MS Excel and the open epi program. The frequency distribution and 95% confidence intervals around the point estimates were derived for categorical variables and the mean (SD) for continuous variables. The student’s t-test and a one way analysis of variance (ANOVA) test was used to compare the MAP by different subgroups. A binomial test of proportion was used to compare women with gestation age specific Mean Uterine Artery PI >95th percentiles by different subgroups. A normal subgroup of the screened population, after excluding data of women aged >35 years, obesity based on BMI, with a past history of PE, or with associated medical co-morbidities, was used to determine the percentile values of MAP and Mean Uterine Artery PI. The percentile distributions of the gestation age specific mean Uterine artery doppler PI was compared with the global reference standard. [13] A bivariate analysis was used to explore the association of MAP >95th percentile and Mean Uterine Artery PI >95th percentile with risk for preterm PE in 1st trimester pregnant women and presented as odds ratio (OR) and 95% confidence intervals around the point estimate. The frequency distribution and 95% CI around point estimates of 1st trimester pregnant women at risk for preterm PE and FGR in the screened population using different cut-off criteria (1 in 50, 1 in 100 and 1 in 150) was determined. A p value <0.05 was considered as statistically significant.
Results
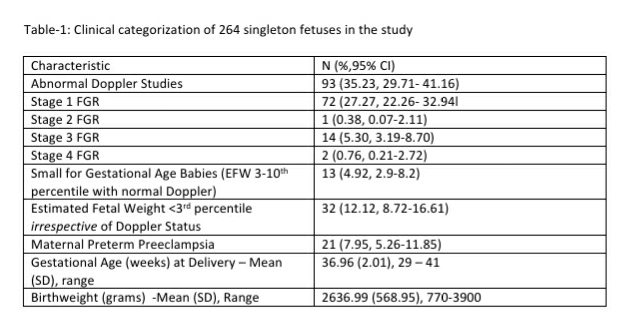
We analyzed records of 750 pregnant women with a singleton fetus screened as part of the Samrakshan program at gestational ages between 11 to 13+6 weeks. The mean (SD) age of women included in the analysis was 28.00 (5.15) years and the mean (SD) body mass index (BMI) was 24.16 (5.00). Most women were screened in the 12th (n=325) and 13th (n=334) gestation week. The majority of women (n=429) were aged 20 to 29 years (see Table -1).

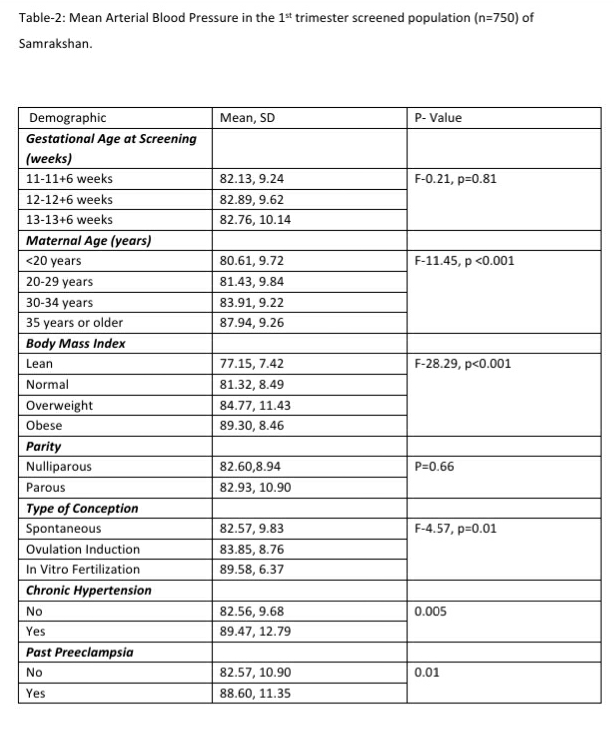
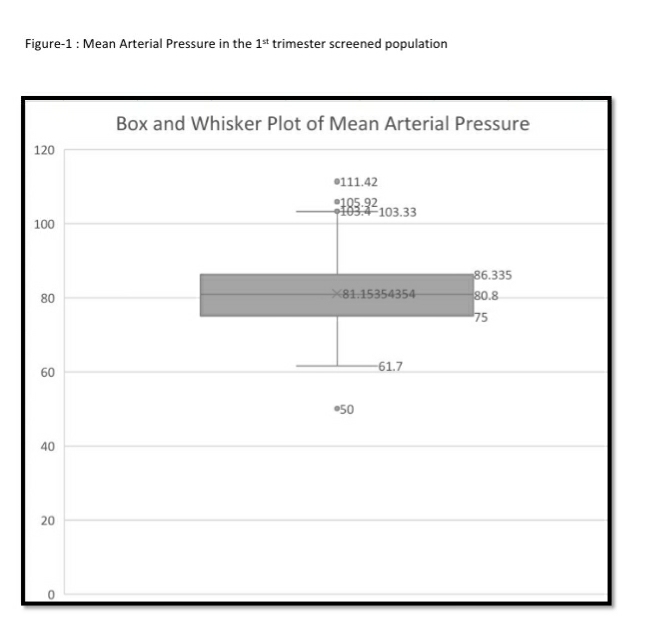
The mean arterial pressure was significantly higher in pregnant women aged 35 years or older, obese women (BMI>30 kg/m2), women who conceived through in vitro fertilization (IVF), women with chronic hypertension and women who had a prior history of PE (see Table-2). The 95th percentile of the MAP distribution in the screened 1st trimester pregnant women with normal parameters was 97, the 50th percentile was 80.8 (see Figure-1) and the 5th percentile was 68.76. A MAP >95th percentile was found in 52 (6.93%, 95% CI: 5.33, 8.98) of the 750 pregnant women screened in the 1st trimester as part of Samrakshan. Table-3 shows the association of MAP >95th percentile with clinico-demographic characteristics.
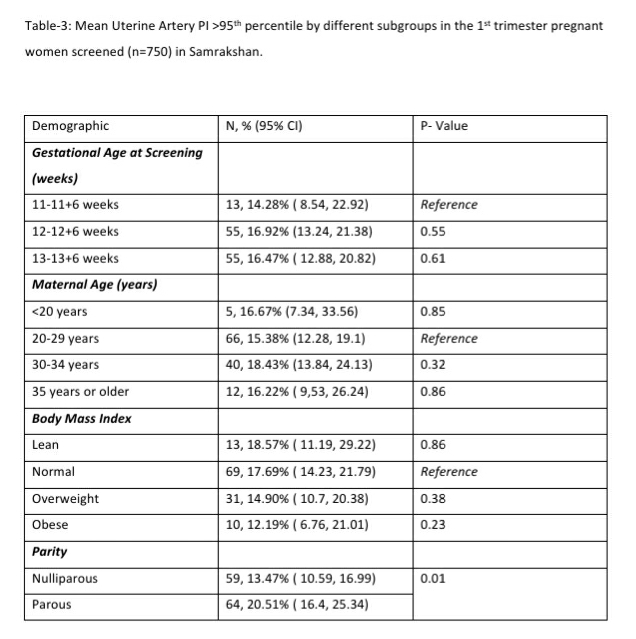
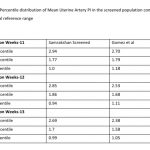
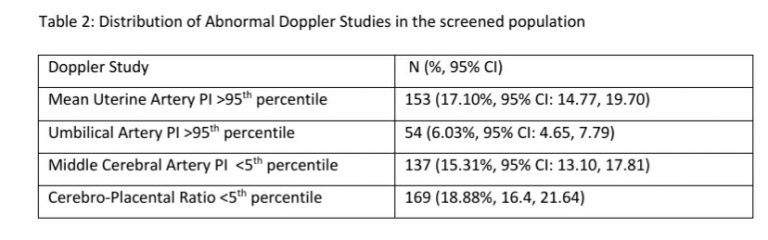
122 women (16.27%, 95% CI: 13.80, 19.08) had a gestation age specific mean uterine artery PI >95th percentile based on the global reference standard. The 95th percentile values of the mean uterine artery PI (derived in a normal subgroup of the screened population) was consistently higher than the global reference standard for gestational ages 11 to 13+6 weeks (see Table-4).
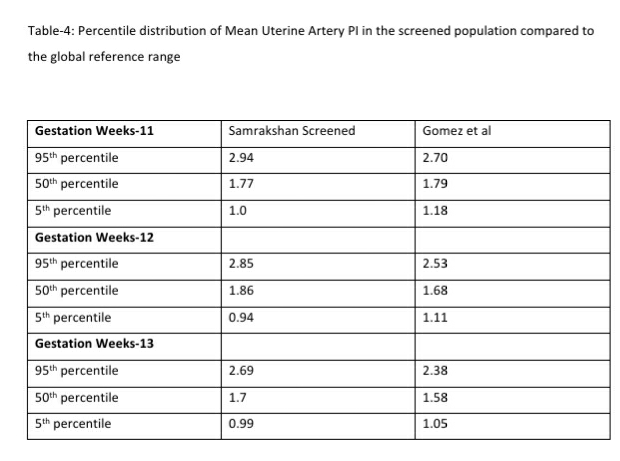
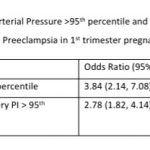
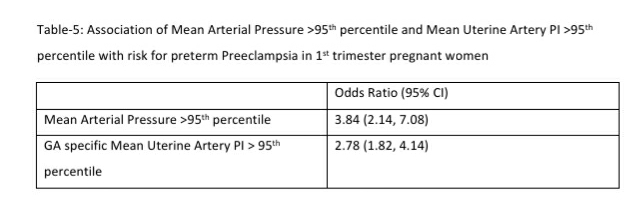
Mean arterial blood pressure >95th percentile and mean uterine artery PI >95th percentile was significantly associated with a higher risk for preterm PE (see Table-5). Table-6 shows the distribution of pregnant women at risk for preterm PE and FGR using different cut-off criteria for risk estimation.
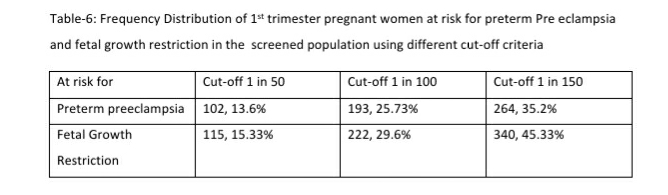
Low Dose Aspirin was recommended for 359 of the 750 pregnant women screened in the 1st trimester. This included 168 (22.4%, 95% CI: 19.56, 25.52) women at high risk (using a 1 in 150 cut off) for both preterm PE and FGR; an additional 96 (12.8%, 95% CI: 10.6, 15.38) women at high risk only for preterm PE and 95 (12.67%, 95% CI: 10.48, 15.24) women at high-risk only for FGR.
Discussion
There were three important aspects that we wanted to explore in the analysis of the baseline results of the 1st trimester protocol that used the evidence based FMF algorithm. The first was to explore the distribution of MAP and mean uterine artery Doppler PI and the magnitude and direction of their association with high risk for preterm PE and FGR. The second aspect of interest was to determine if the percentile values of the mean uterine artery Doppler PI in this population is similar to the global gestation age specific reference range. The third aspect was to determine the difference in the frequency distribution of women identified as at high risk, if we use different cut-offs, 1 in 50, 1 in 100 or 1 in 150. The process of determining the diagnostic effectiveness of the different cut-off is ongoing and will be presented separately.
MAP is not a routine measure in most 1st trimester screening processes in India except in centers that use a customized risk estimation protocol. Understanding the magnitude and direction of the association of MAP with high risk for preterm PE in our population is an important first step. This may encourage more people to measure MAP using a standard protocol with digital blood pressure measures. We found a MAP >95th percentile to be significantly associated with a high risk of preterm PE in this population. Additionally, we found MAP to be associated with several subgroups- the elderly, women with a BMI of 30 or more, women conceiving with the help of IVF, and women with chronic hypertension or a past history of PE. One important consideration is whether MAP should be measured for every pregnant or can be limited to certain subgroups given the large number of pregnant women in India. It is important to study further the percentile distribution of MAP from diverse populations of India and the association of different percentiles with high risk for preterm PE. At this stage, Samrakshan recommends the measurement of MAP in every pregnant woman as it forms an integral part of the personalized risk assessment for preterm PE using the FMF algorithm.
The measurement of mean uterine artery Doppler PI is to be integrated into routine 1st trimester screening protocols. 1st trimester mean uterine artery PI >95th percentile was associated with a high risk of preterm PE indicating the importance of this measure. Several studies have reported a relatively poor performance of risk estimators like clinico-demographic details especially when used in isolation. There is a considerable body of evidence in the global literature on the improved efficacy of 1st trimester screening protocols that integrate MAP and mean uterine artery PI.[4] Integrating biochemical tests will increase the efficacy further [4,14,15] but is not possible yet on a large scale in India.
Low dose aspirin can be started early in the 1st trimester if we identify pregnant women at risk for preterm PE and FGR. The effectiveness of low dose aspirin is optimal when started between 11-14 gestation weeks and reduces if started at later gestational ages.[4] The determination of who receives low dose aspirin is based on the cut-off used for the risk estimation. We found that 35.2% of 1st trimester women may need low dose aspirin if we use a cut-off of 1 in 150 as risk for preterm PE. If we consider the same risk cut-off for FGR, the proportion of women needing low dose aspirin increases to 45.3%. These numbers, although large, have to be viewed in the context of the magnitude of FGR in India. We have earlier reported that an abnormal Doppler study was present in 35.23% of the Samrakshan screened population that gave birth.[11] Reported estimates of FGR and small for gestational age babies are also on the higher side (43-54%) in India. [16-20]
The proportion of women that are considered as high risk shows a significant reduction from 35.2% to 25.73% if we use a cut-off of 1 in 100 for preterm PE and from 45.3% to 29.6% for FGR. These proportions fall significantly further when we use a risk of 1 in 50, to 13.6% for preterm PE and 15.33% for FGR. These are significant reductions that translate to very large absolute numbers when we consider the large number of pregnancies in India. Changing the cut-off to 1 in 50 or 1 in 100 reduces significantly the number of pregnancies to be closely monitored and the number of women that need low dose aspirin.
However, there are several aspects to consider before choosing a revised cut-off. One, should we consider the effectiveness of Aspirin in the prevention of FGR? Is the evidence of effectiveness of aspirin as robust for FGR, especially mild to moderate FGR, when compared with preterm PE? [21,22] We found that 95 (12.67%) of the 359 women receiving aspirin were identified as at high risk for FGR without an accompanying high risk for PE. Should we give these women low dose aspirin?
We have earlier reported an incidence of 9.85% for PE, similar to the nationally reported estimates of 8-10% in India. Using a cut-off of 1 in 50 identifies 13.6% of 1st trimester screened pregnant women as at high risk for preterm PE and is closer to the known estimates of incidence of PE. However, using a 1 in 50 cut-off results in the identification of only 15.33% women as high risk for FGR, which is significantly lower than the 35.23% pregnant women with abnormal Doppler study that we found in the Samrakshan screened population.[11] Is this, then, a reasonable estimate to use? Will we miss a significant proportion of at risk for FGR fetuses, which is one of the major causes for perinatal mortality in India, with a 1 in 50 cut-off?
The potential miss of apparently large numbers of FGR has to be balanced with the severity of the stage of FGR and the robustness of the evidence for aspirin in FGR. It is more likely the 1 in 50 cut-off identifies the more severe FGR at higher risk for perinatal mortality. What we may miss picking up are probably the milder cases that may have relatively lower risk of mortality. To answer this, we need to consider the progression from the 1st trimester to the development and stage of FGR and childbirth outcomes. We also need to determine the diagnostic effectiveness of low dose aspirin at different cut-offs. Currently, we do not have the answers to these questions from diverse populations in India.
It is also important to explore the effectiveness of the algorithm and the parameters underpinning the algorithm used to determine the personalized risk. The measures and definitions of clinico-demographic details may not differ much and thus may not contribute much noise to the algorithm. Measuring MAP using a non-standardized protocol including seated position, measurements in both arms and use of a digital apparatus is an element that can introduce variation in the risk estimates. Potential differences of MAP percentile values in regional populations of India is another element that can introduce variation in the risk estimate. Differences in the percentile values of mean uterine artery PI compared to the gestational age specific global reference standards, or by regional populations, can introduce variations in the risk estimates. These are areas that can impact the diagnostic effectiveness of the algorithm and different cut-offs and need further study.
A very important consideration for preventative services, in a country like India, is the uptake of antenatal care services and follow up rates. Although improving, the National Family Health Survey (NFHS)-4 reported a less than 60% uptake of antenatal care services in the 1st trimester. [2] The proportion of women having an antenatal check-up in all trimesters is improving but is still suboptimal. [2] The consequences of PE and FGR on perinatal health of the mother and fetus are grave, in the short and long term. We have to balance the consequences, uptake and use of antenatal care services, magnitude of FGR, access to neonatal or maternal ICU care in rural areas and the potential benefits from aspirin. Using a cut-off of 1 in 150 to estimate risk looks reasonable when we consider all these factors. A key factor is the low cost and easy availability of aspirin. If the preventive measure was costlier and not easily available, restricting to a more severe population, a 1 in 50 cut-off, may have been more reasonable.
Should all components of the algorithm be used or can risk estimates be done by using clinico-demographic details alone, or by measuring MAP alone or by measuring mean uterine artery PI percentile values in isolation? There is a large body of evidence that has looked at this question and hence, we did not consider exploring this again. [4] Data from a large cohort of prospectively followed singleton pregnancies showed that, at a false‐positive rate of 10%, maternal factors alone (including age, weight, ethnic origin, reproductive and medical history and smoking) could predict only 49% of PE < 37 weeks. [4] Screening by first‐trimester uterine artery PI > 90th centile detects only 48% of women who will develop early PE and 26% of those who will develop any PE, for a 10% screen‐positive rate.[4]. The recommendation is to use a multifactorial screening model instead of a standalone use of uterine artery PI considering that maternal factors can affect uterine artery PI. [4] A combination of maternal clinico-demographic factors, MAP, uterine artery Doppler at 11–13 weeks appears to be more efficient as a screening model for identification of women at risk of PE. The efficiency of this model is further enhanced with the inclusion of PIGF where available. Samrakshan recommends that all elements of the algorithm are used in combination and not in isolation.
The dose of low dose aspirin is an aspect to be addressed. Samrakshan follows the evidence from the Aspirin for Evidence-Based Preeclampsia Prevention (ASPRE) trial and recommends 150 mg once daily at bed time.[5,6] Although ISUOG [4] and FIGO guidelines [23] endorse 150 mg, several obstetricians still prefer lower doses. We found many obstetricians preferring a dosage of 75 or 81 mg and without specifying bedtime as the appropriate time to take low dose aspirin. Appropriate dosage at the appropriate time and compliance are factors that will influence outcomes assessment of the effectiveness of Aspirin. The current practice in India, by Fetal Radiologists, is to make a recommendation to start aspirin to the primary physician. It is debatable whether radiologists should prescribe low dose aspirin as soon as risk is identified in the 1st trimester to reduce potential delays to start aspirin.
At this stage, Samrakshan is exploring evidence around these questions and does not have any prescriptive answers yet. The baseline analysis of the 1st trimester screening protocol brings up several important and priority questions or directions for Fetal Radiology research in India. This has to be studied in diverse populations within India, accounting for regional diversity as well as urban rural divides, to be truly applicable at the national level.
References
- National Health Portal, India. Accessed online at https://www.nhp.gov.in/disease/gynaecology-and-obstetrics/preeclampsia
- International Institute for Population Sciences (IIPS) and ICF. 2017. National Family Health Survey (NFHS-4) 2015-16: India. Mumbai: IIPS
- Million Death Study Collaborators, Bassani DG, Kumar R, et al. Causes of neonatal and child mortality in India: a nationally representative mortality survey. Lancet. 2010; 376:1853–1860
- Sotiriadis A, Hernandez‐Andrade E, da Silva Costa F, et al. ISUOG Practice Guidelines: Role of ultrasound in screening for and follow‐up of pre‐eclampsia. Ultrasound Obstet Gynecol. 2019; 53: 7– 22.
- Rolnik DL, Wright D, Poon LCY, et al. ASPRE trial: Performance of screening for preterm pre‐eclampsia. Ultrasound Obstet Gynecol. 2017;50:492–495.
- Rolnik DL, Wright D, Poon LC, et al. Aspirin versus placebo in pregnancies at high risk for preterm preeclampsia. N Engl J Med. 2017;377:613–622.
- Choorakuttil RM, Patel H, Bavaharan R, Devarajan P, Kanhirat S, Shenoy RS, Tiwari OP, Sodani RK, Sharma LK, Nirmalan PK. Samrakshan: An Indian radiological and imaging association program to reduce perinatal mortality in India. Indian J Radiol Imaging 2019;29:412-7
- Choorakuttil RM, Devarajan P, Rajalingam B, Jain N, Sharma LK, Nagar S, et al. Samrakshan: Rationale for universal 1st trimester screening to identify pregnant women at risk for preterm preeclampsia. Accessed online from http://fetalradiology.in/2019/11/14/2908/ on March 4, 2020
- Anjali G, Renu S, Sharma LK, Akanksha B, Bavaharan R, Shilpa RS, at al. Colour Doppler studies identify more fetuses at risk for compromise in the third trimester: Preliminary results from Samrakshan India. Journal of Fetal Radiology. Accessed online from http://fetalradiology.in/2020/02/09/colour-doppler-studies-identify-more-fetuses-at-risk-for-compromise-in-the-third-trimester-preliminary-results-from-samrakshan-india/ on March 1, 2020
- Neelam Jain, Prashant M Onkar, Sunitha Pradeep, Devarajan P, Anjali Gupta, Lalit K Sharma,at al. 2nd trimester screening to identify pregnancies at risk for preterm preeclampsia and fetal growth restriction: Preliminary results from Samrakshan India. Journal of Fetal Radiology. Accessed online from http://fetalradiology.in/2020/02/24/2nd-trimester-screening-to-identify-pregnancies-at-risk-for-preterm-preeclampsia-and-fetal-growth-restriction-preliminary-results-from-samrakshan-india/ on March 1, 2020
- Rijo M Choorakuttil, Vanaj Mathur, Lalit K Sharma, Anjali Gupta, Shilpa Satarkar, Renu Sharma, at al. Baseline information on childbirth outcomes among pregnant women screened in the Samrakshan program, India. Accessed online on March 10,2020 from http://fetalradiology.in/2020/03/04/baseline-information-on-childbirth-outcomes-among-pregnant-women-screened-in-the-samrakshan-program-india/
- Poon LC, Zymeri NA, Zamprakou A, Syngelaki A, Nicolaides KH. Protocol for measurement of mean arterial pressure at 11‐13 weeks’ gestation. Fetal Diagn Ther. 2012; 31: 42– 48.
- Gómez, O., Figueras, F., Fernández, S., Bennasar, M., Martínez, J.M., Puerto, B. and Gratacós, E. (2008), Reference ranges for uterine artery mean pulsatility index at 11–41 weeks of gestation. Ultrasound Obstet Gynecol, 32: 128-132. doi:10.1002/uog.5315
- Zhong Y, Zhu F, Ding Y. Serum screening in first trimester to predict pre‐eclampsia, small for gestational age and preterm delivery: Systematic review and meta‐analysis. BMC Pregnancy Childbirth. 2015;15:191.
- Morris RK, Bilagi A, Devani P, Kilby MD. Association of serum PAPP‐A levels in first trimester with small for gestational age and adverse pregnancy outcomes: Systematic review and meta‐analysis. Prenat Diagn. 2017; 37: 253– 265
- Antonisamy B, Sivaram M, Richard J, Rao PSS. Trends in Intra-uterine Growth of Single Live Births in Southern India. J Trop Pediatr. 1996. 339–341
- Pinheiro A, David A, Joseph B. Pregnancy weight gain and its correlation to birth weight. Indian J Med Sci. 2001;55:266–270.
- Maternal anthropometry and pregnancy outcomes. A WHO Collaborative Study: Introduction. Bull World Health Organ. 1995. S1–98.
- S. Murki, D. Sharma. Intrauterine Growth Retardation – A Review Article. J Neonatal Biol 2014, 3:3. DOI: 10.4172/2167-0897.1000135
- Deepak Sharma, Sweta Shastri, Nazanin Farahbakhsh & Pradeep Sharma (2016) Intrauterine growth restriction – part 1, The Journal of Maternal-Fetal & Neonatal Medicine, 29:24, 3977-3987.
- Low-dose aspirin in prevention and treatment of intrauterine growth retardation and pregnancy-induced hypertension. Italian study of aspirin in pregnancy. Lancet. 1993;341(8842):396–400.
- Roberge S, Nicolaides K, Demers S, Hyett J, Chaillet N, Bujold E. The role of aspirin dose on the prevention of preeclampsia and fetal growth restriction: systematic review and meta-analysis. Am J Obstet Gynecol. 2017;216(2):110–120.e6. doi:10.1016/j.ajog.2016.09.076
- Poon, L.C., Shennan, A., Hyett, J.A., Kapur, A., Hadar, E., Divakar, H., McAuliffe, F., da, Silva Costa, F., von, Dadelszen, P., McIntyre, H.D., Kihara, A.B., Di Renzo, G.C., Romero, R., D’Alton, M., Berghella, V., Nicolaides, K.H. and Hod, M. (2019), The International Federation of Gynecology and Obstetrics (FIGO) initiative on pre‐eclampsia: A pragmatic guide for first‐trimester screening and prevention. Int J Gynecol Obstet, 145: 1-33. doi:10.1002/ijgo.12802